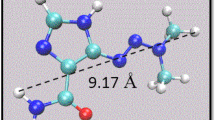
Abstract
The adsorption of the anticancer drugs sorafenib (SF), streptozotocin (STZ), and sunitinib (STB) on pristine and functionalized carbon nanotubes (FCNTs, functionalized with valine or phenylalanine moieties) was investigated using molecular dynamics simulation. Descriptors such as the van der Waals (vdW) energy, the number of hydrogen bonds, and the radial distribution function were considered. It was found that the type of functional group on the nanotube is a key influence on the vdW interaction energy between a drug molecule and a nanotube. In addition, the positions of the functional groups on a nanotube are a key influence on the adsorption of drug molecules on its surface. Our study indicated that the adsorption of STZ on CNT/FCNTs involves a partial π–π interaction and hydrogen bonding, whereas SF and STB are adsorbed on CNT/FCNTs through π–π stacking and hydrogen bonding. Our results suggest that altering the functionalization of the nanotube surface can affect the drug–nanotube interaction. The results reported here should aid attempts to optimize the design of novel CNT-based drug carriers.











Similar content being viewed by others
References
Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109:3012–3043
Kushwaha S, Rastogi A, Rai A et al (2012) Novel drug delivery system for anticancer drug: a review. Int J Pharm Tech Res 4:542–553
Vashist SK, Zheng D, Pastorin G et al (2011) Delivery of drugs and biomolecules using carbon nanotubes. Carbon 49:4077–4097
Wilhelm S, Carter C, Lynch M et al (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5:835–844
Smalley KS, Xiao M, Villanueva J et al (2009) CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene 28:85–94
Wang XQ, Fan JM, Liu YO et al (2011) Bioavailability and pharmacokinetics of sorafenib suspension, nanoparticles and nanomatrix for oral administration to rat. Int J Pharm 419:339–346
Sun L, Liang C, Shirazian S et al (2003) Discovery of 5-[5-fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3 carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase J Med Chem 46:1116–1119
Raymond E, Dahan L, Raoul JL et al (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501–513
Bagcchi S (2014) Sunitinib still first-line therapy for metastatic renal cancer. Lancet Oncol 15:e420
Moertel CG, Johnson CM, McKusick MA et al (1994) The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med 120:302–309
Schlumberger M, Abdelmoumene N, Delisle MJ et al (1995) Treatment of advanced medullary thyroid cancer with an alternating combination of 5 FU streptozocin and 5 FU-dacarbazine. Br J Cancer 71:363–365
Broder LE, Carter SK (1973) Pancreatic islet cell carcinoma: II. Results of therapy with streptozotocin in 52 patients. Ann Intern Med 79:108–118
McDevitt MR, Chattopadhyay D, Kappel BJ et al (2007) Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med 48:1180–1189
Liu Z, Chen K, Davis C et al (2008) Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 68:6652–6660
Wong BS, Yoong SL, Jagusiak A et al (2013) Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev 65:1964–2015
Poland CA, Duffin R, Kinloch I et al (2008) Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3:423–428
Liu X, Marangon I, Melinte G et al (2014) Design of covalently functionalized carbon nanotubes filled with metal oxide nanoparticles for imaging, therapy, and magnetic manipulation. ACS Nano 8:11290–11304
Singh R, Torti SV (2013) Carbon nanotubes in hyperthermia therapy. Adv Drug Deliv Rev 65:2045–2060
Liu J, Cui L, Losic D (2013) Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater 9:9243–9257
Kim H, Lee D, Kim J et al (2013) Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano 7:6735–6746
Kurapati R, Raichur AM (2013) Near-infrared light-responsive graphene oxide composite multilayer capsules: a novel route for remote controlled drug delivery. Chem Commun 49:734–736
Mertz D, Sandre O, Begin-Colin S (2017) Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim Biophys Acta Gen Subj 1861:1617–1641
Liu Z, Tabakman S, Welsher K et al (2009) Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res 2:85–120
Colvin VL (2003) The potential environmental impact of engineered nanomaterials. Nat Biotechnol 21:1166–1170
Delogu LG, Venturelli E, Manetti R et al (2012) Ex vivo impact of functionalized carbon nanotubes on human immune cells. Nanomedicine 7:231–243
Bianco A, Kostarelos K, Prato M (2005) Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 9:674–679
Liu P (2013) Modification strategies for carbon nanotubes as a drug delivery system. Ind Eng Chem Res 52:13517–13527
Rajarajeswari M, Iyakutti K, Kawazoe Y (2011) Interaction of valine and valine radicals with single-walled carbon nanotube (5, 0). Chem Phys Lett 511:299–303
Chawla S, Pundir CS (2011) An electrochemical biosensor for fructosyl valine for glycosylated hemoglobin detection based on core-shell magnetic bionanoparticles. Biosens Bioelectron 26:3438–3443
Dubey SK, Sharma AK, Narain U, Misra K, Pati U (2008) Design, synthesis and characterization of some bioactive conjugates of curcumin with glycine, glutamic acid, valine and demethylenated piperic acid and study of their antimicrobial and antiproliferative properties. Eur J Med Chem 43:1837–1846
Mahalakshmi R, Jesuraja SX, Jerome Das S (2006) Growth and characterization of L-phenylalanine. Cryst Res Technol 41:780–783
Jeong JS, Sim HJ, Lee YM et al (2009) Determination of phenylalanine in blood by high-performance anionexchange chromatography-pulsed amperometric detection to diagnose phenylketonuria. J Chromatogr A 1216:5709–5714
Kand’ar R, Zakova P (2009) Determination of phenylalanine and tyrosine in plasma and dried blood samples using HPLC with fluorescence detection. J Chromatogr B 877:3926–3929
Deborah M, Jawahar A, Mathavan T et al (2015) Spectroscopic studies on valine-functionalized single-walled carbon nanotubes. Fuller Nanotub Car N 23:649–657
Thakare VS, Das M, al JAK (2010) Carbon nanotubes in cancer theragnosis. Nanomedicine 5:1277–1301
Mallakpour S, Abdolmaleki A, Borandeh S (2014) L-phenylalanine amino acid functionalized multi walled carbon nanotube (MWCNT) as a reinforced filler for improving mechanical and morphological properties of poly(vinyl alcohol)/MWCNT composite. Prog Org Coat 77:1966–1971
Li J, Grennberg H (2006) Microwave-assisted covalent sidewall functionalization of multiwalled carbon nanotubes. Chem Eur J 12:3869–3875
Oyetade OA, Martincigh BS, Skelton AA (2018) Interplay between electrostatic and hydrophobic interactions in the pH-dependent adsorption of ibuprofen onto acid-functionalized multiwalled carbon nanotubes. J Phys Chem C 122:22556–22568
Wolski P, Nieszporek K, Panczyk T (2017) Pegylated and folic acid functionalized carbon nanotubes as pH controlled carriers of doxorubicin. Molecular dynamics analysis of the stability and drug release mechanism. Phys Chem Chem Phys 19:9300–9312
Li Z, Tozer T, Alisaraie L (2016) Molecular dynamics studies for optimization of noncovalent loading of vinblastine on single-walled carbon nanotube. J Phys Chem C 120:4061–4070
Abdolmaleki A, Mallakpour S, Borandeh S (2013) Amino acid-functionalized multi-walled carbon nanotubes for improving compatibility with chiral poly(amide-ester-imide) containing l-phenylalanine and l-tyrosine linkages. Appl Surf Sci 287:117–123
Arsano I, Li S, Wasala M, Tsige M et al (2018) Molecular dynamics of benzoic acid adsorption on carboxylated carbon nanotubes. APS Meeting Abstr F17.013
Ajori S, Ansari R, Darvizeh M (2016) On the vibrational behavior of single-and double-walled carbon nanotubes under the physical adsorption of biomolecules in the aqueous environment: a molecular dynamics study. J Mol Model 22:62
Ansari R, Rouhi S, Ajori S (2018) Molecular dynamics simulations of the polymer/amine functionalized single-walled carbon nanotubes interactions. Appl Surf Sci 455:171–180
Al-Qattan MN, Deb PK, Tekade RK (2017) Molecular dynamics simulation strategies for designing carbon-nanotube-based targeted drug delivery. Drug Discov Today
Ansari R, Ajori S, Malakpour S (2016) Prediction of structural and mechanical properties of atom-decorated porous graphene via density functional calculations. Eur Phys J-Appl Phys 74:10401
Anota EC, Villanueva MS, Shakerzadeh E, Castro M (2018) Adsorption and possible dissociation of glucose by the [BN fullerene-B6]− magnetic nanocomposite. In silico studies. Appl Nanosci 8:455–465
Hooshmand S, Es’haghi Z (2018) Hydrophilic modified magnetic multi-walled carbon nanotube for dispersive solid/liquid phase microextraction of sunitinib in human samples. Anal Biochem 542:76–83
Martins-Júnior PA, Alcântara CE, Resende RR, Ferreira AJ (2013) Carbon nanotubes: directions and perspectives in oral regenerative medicine. J Dent Res 92:575–583
Karadas-Bakirhan N, Patris S, Ozkan SA, Can A et al (2016) Determination of the anticancer drug sorafenib in serum by adsorptive stripping differential pulse voltammetry using a chitosan/multiwall carbon nanotube modified glassy carbon electrode. Electroanalysis 28:358–365
Frey JT, Doren DJ (2011) TubeGen3.4 (web interface). J.T. Frey and D.J. Doren, University of Delaware, Newark. http://turin.nss.udel.edu/research/tubegenonline.html
Hess B, Kutzner C, Van Der Spoel D et al (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Ricci CG, de Andrade AS, Mottin M et al (2010) Molecular dynamics of DNA: comparison of force fields and terminal nucleotide definitions. J Phys Chem B 114:9882–9893
Zoete V, Cuendet MA, Grosdidier A et al (2011) SwissParam: a fast force field generation tool for small organic molecules. J Comput Chem 32:2359–2368
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: an N·log (N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Berendsen HJ, Postma JV, van Gunsteren WF et al (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Schmidt MW, Baldridge KK, Boatz JA et al (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Hasanzade Z, Raissi H (2017) Investigation of graphene-based nanomaterial as nanocarrier for adsorption of paclitaxel anticancer drug: a molecular dynamics simulation study. J Mol Model 23:36–43
Hasanzade Z, Raissi H (2017) Solvent/co-solvent effects on the electronic properties and adsorption mechanism of anticancer drug thioguanine on graphene oxide surface as a nanocarrier: density functional theory investigation and a molecular dynamics. Appl Surf Sci 422:1030–1041
Hasanzade Z, Raissi H (2018) Density functional theory calculations and molecular dynamics simulations of the adsorption of ellipticine anticancer drug on graphene oxide surface in aqueous medium as well as under controlled pH conditions. J Mol Liq 255:269–278
Hasanzade Z, Raissi H (2018) Assessment of the chitosan-functionalized graphene oxide as a carrier for loading thioguanine, an antitumor drug and effect of urea on adsorption process: combination of DFT computational and molecular dynamics simulation studies. J Biomol Struct Dyn (accepted)
Hashemzadeh H, Raissi H (2018) Covalent organic framework as smart and high efficient carrier for anticancer drug delivery: a DFT calculations and molecular dynamics simulation study. J Phys D Appl Phys 51:345401
Choudhary V, Gupta A (2011) Polymer/carbon nanotube nanocomposites. In: carbon nanotubes–polymer nanocomposites. InTechOpen, London
Aihara JI (1994) Lack of superaromaticity in carbon nanotubes. J Phys Chem 98:9773–9776
Hashemzadeh H, Raissi H (2017) The functionalization of carbon nanotubes to enhance the efficacy of the anticancer drug paclitaxel: a molecular dynamics simulation study. J Mol Model 23:222
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer J Comput Chem 33:580–592
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 64284 kb)
Rights and permissions
About this article
Cite this article
Dehneshin, N., Raissi, H., Hasanzade, Z. et al. Using molecular dynamics simulation to explore the binding of the three potent anticancer drugs sorafenib, streptozotocin, and sunitinib to functionalized carbon nanotubes. J Mol Model 25, 159 (2019). https://doi.org/10.1007/s00894-019-4024-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4024-5