Abstract
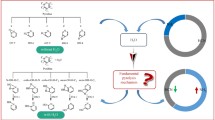
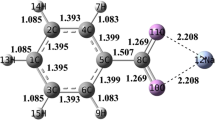
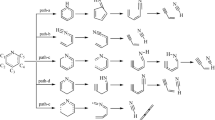
The catalytic pyrolysis pathways of carbonyl compounds in coal were systematically studied using density functional theory (DFT), with benzaldehyde (C6H5CHO) employed as a coal-based model compound and ZnO, γ-Al2O3, and CaO as catalysts. The results show that the products of both pyrolysis and catalytic pyrolysis are C6H6 and CO. However, the presence of any of the catalysts changes the reaction pathway and reduces the energy barrier, indicating that these catalysts promote C6H5CHO decomposition.

The presence of catalysts changes the reaction pathway and the energy barrier decreases in the order Ea (no catalyst)> Ea (CaO)> Ea (γ-Al2O3)> Ea (ZnO), indicating that these catalysts promote C6H5CHO decomposition.




Similar content being viewed by others
References
Reichel D, Siegl S, Neubert C, Krzack S (2015) Determination of pyrolysis behavior of brown coal in a pressurized drop tube reactor. Fuel 158:983–998
Ding L, Zhou ZJ, Guo QH, Huo W, Yu GS (2015) Catalytic effects of Na2CO3 additive on coal pyrolysis and gasification. Fuel 142:134–144
Han JZ, Wang XD, Yue JR, Gao SQ, Xu GW (2014) Catalytic upgrading of coal pyrolysis tar over char-based catalysts. Fuel Process Technol 122:98–106
Li SS, Ma XQ (2016) Catalytic characteristics of the pyrolysis of lignite over oil shale chars. Appl Therm Eng 106:865–874
Sharma A, Matsumura A, Takanohashi (2015) Effect of CO2 addition on gas composition of synthesis gas from catalytic gasification of low rank coals. Fuel 152:13–18
Pütün E (2010) Catalytic pyrolysis of biomass: effects of pyrolysis temperature, sweeping gas flow rate and MgO catalyst. Energy 35:2761–2766
Zhu TY, Zhang SY, Huang JJ, Wang Y (2000) Effect of calcium oxide on pyrolysis of coal in a fluidized bed. Fuel Process Technol 64:271–284
OÖztaş NA, Yürüm Y (2000) Pyrolysis of Turkish Zonguldak bituminous coal. Part 1. Effect of mineral matter. Fuel 79:1221–1227
Abbasi-Atibeh E, Yozgatligil A (2014) A study on the effects of catalysts on pyrolysis and combustion characteristics of Turkish lignite in oxy-fuel conditions. Fuel 115:841–849
Cheng XH, He XM, Chen C, Yi S (2015) Influence of Fe2O3/CaO catalysts on the pyrolysis products of low-rank coal. Energy Technol 3:1068–1071
Yu JL, Tian FJ, Chow MC, McKenzie LJ, Li CZ (2006) Effect of iron on the gasification of Victorian brown coal with steam enhancement of hydrogen production. Fuel 85:127–133
Li Y, Amin MN, Lu XM, Li CS, Ren FQ, Zhang SJ (2016) Pyrolysis and catalytic upgrading of low-rank coal using a NiO/MgO–Al2O3 catalyst. Chem Eng Sci 155:194–200
Jolly R, Charcosset H, Boudou JP, Guet JM (1988) Catalytic effect of ZnCl2 during coal pyrolysis. Fuel Process Technol 20:51–60
Kandiyoti R, Lazaridis JI, Dyrvold B, Weerasinghe CR (1984) Pyrolysis of a ZnCl2-impregnated coal in an inert atmosphere. Fuel 63:1583–1587
Liu QR, Hu HQ, Zhou Q, Zhu SW, Chen GH (2004) Effect of inorganic matter on reactivity and kinetics of coal pyrolysis. Fuel 83:713–718
Orrego-Ruiz JA, Cabanzo R, Mejía-Ospino E (2011) Study of Colombian coals using photoacoustic Fourier transform infrared spectroscopy. Int J Coal Geol 85:307–310
Solomon PR, Hamblen DG, Carangelo RM, Serio MA, Deshpande GV (1988) General model of coal devolatilization. Energy Fuel 2:405–422
Solomon PR, Serio MA, Carangelo RM, Bassilakis R (1990) Analysis of the Argonne premium coal samples by thermogravimetric Fourier transform infrared spectroscopy. Energy Fuel 4:319–333
Odeh A (2015) Oualitative and quantitative ATR-FTIR analysis and its application to coal char of different ranks. J Fuel Chem Technol 43:129–137
Wang BJ, Zhang RG, Ling LX (2012) Quantum chemistry study on the pyrolysis mechanisms of coal-related model compounds. In: DaCosta H, Fan M (eds) Rate constant calculation for thermal reactions: methods and applications. Wiley, Hoboken, pp 239–282
Li L, Fan HJ, Hu HQ (2015) A theoretical study on bond dissociation enthalpies of coal based model compounds. Fuel 153:70–77
Murata S, Hosokawa M, Kidena K, Nomura M (2000) Analysis of oxygen-functional groups in brown coals. Fuel Process Technol 67:231–243
Li G, Li L, Shi L, Jin L, Tang Z, Fan H, Hu H (2014) Experimental and theoretical study on the pyrolysis mechanism of three coal-based model compounds. Energy Fuel 28:980–986
Liu SY, Zhang ZQ, Wang HF (2012) Quantum chemical investigation of the thermal pyrolysis reactions of the carboxylic group in a brown coal model. J Mol Model 18:359–365
Pecullan M, Brezinsky K, Glassman I (1997) Pyrolysis and oxidation of anisole near 1000 K. J Phys Chem A 101:3305–3316
Wang MF, Zuo Z, Ren RP, Gao ZH, Huang W (2016) Theoretical study on catalytic pyrolysis of benzoic acid as a coal-based model compound. Energy Fuel 30:2833–2840
Bates CH, White WB, Roy R (1962) New high-pressure polymorph of zinc oxide. Science 137:993
Digne M, Sautet P, Raybaud P, Euzen P, Toulhoat H (2004) Use of DFT to achieve a rational understanding of acid-basic properties of γ-alumina surfaces. J Catal 226:54–68
Zhao S, Ma XD, Pang Q, Sun HW, Wang GC (2014) Dissociative adsorption of 2,3,7,8-TCDD on the surfaces of typical metal oxides: a first-principles density functional theory study. Phys Chem Chem Phys 16:5553–5562
Piskorz W, Zasada F, Stelmachowski P, Kotarba A, Sojka Z (2013) DFT Modeling of reaction mechanism and ab initio microkinetics of catalytic N2O decomposition over alkaline earth oxides: from molecular orbital picture account to simulation of transient and stationary rate profiles. J Phys Chem C 117:18488–18501
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133–A1138
Ordejón P, Artacho E, Soler JM (1996) Self-consistent order-N density-functional calculations for very large systems. Phys Rev B 53:R10441
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871
Halgren TA, Lipscomb WN (1977) The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem Phys Lett 49:225–232
Yang YX, Evans J, Rodriguez JA, White MG, Liu P (2010) Fundamental studies of methanol synthesis from CO2 hydrogenation on Cu(111), Cu clusters, and Cu/ZnO(000\( \overline{1} \)). Phys Chem Chem Phys 12:9909–9917
Liu YM, Liu JT, Liu SZ, Li J, Gao ZH, Zuo ZJ, Huang W (2017) Reaction mechanisms of methanol synthesis from CO/CO2 hydrogenation on Cu2O(111): comparison with Cu(111). J CO2 Util 20:59–65
Zuo ZJ, Wang L, Han PD, Huang W (2014) Insights into the reaction mechanisms of methanol decomposition, methanol oxidation and steam reforming of methanol on Cu(111): a density functional theory study. Int J Hydrogen Energy 39:1664–1679
Naeemullah, Murtaza G, Khenata R, Safeer A, Alahmed ZA, Omran SB (2014) Shift of band gap from indirect to direct and optical response of CaO by doping S, Se, Te. Comput Mater Sci 91:43–49
Kobayashi A, Sankey OF, Dow JD (1983) Deep energy levels of defects in the wurtzite semiconductors AIN, CdS, CdSe, ZnS, and ZnO. Phys Rev B 28:946–956
Gallas MR, Piermarini GJ (1994) Bulk modulus and Young's modulus of nanocrystalline γ-alumina. J Am Ceram Soc 77:2917–2920
Calzolari A, Ruini A, Catellani A (2011) Anchor group versus conjugation: toward the gap-state engineering of functionalized ZnO (10\( \overline{1} \)0) surface for optoelectronic applications. J Am Chem Soc 133:5893–5899
Zuo ZJ, Ramírez PJ, Senanayake SD, Liu P, Rodriguez JA (2016) Low-temperature conversion of methane to methanol on CeO x /Cu2O catalysts: water controlled activation of the C−H bond. J Am Chem Soc 138:13810–13813
Liu ZM, Ma LL, Junaid ASM (2010) NO and NO2 adsorption on Al2O3 and Ga modified Al2O3 surfaces: a density functional theory study. J Phys Chem C 114:4445–4450
Liu JT, Wang MF, Gao ZH, Zuo ZJ, Huang W(2018) The role of catalysts in the decomposition of phenoxy compounds in coal: A density functional theory study. Appl Surf Sci 428:541-548
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (21776197), the Shanxi Province Science Foundation for Youths (201701D211003), the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi, and the Key Project of Basic Industrial Research of Shanxi (201603D121014).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 101 kb)
Rights and permissions
About this article
Cite this article
Cui, LP., Liu, JT., Liu, SZ. et al. A DFT study of the catalytic pyrolysis of benzaldehyde on ZnO, γ-Al2O3, and CaO models. J Mol Model 24, 65 (2018). https://doi.org/10.1007/s00894-018-3587-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3587-x