Abstract
The mechanistic details of N-heterocyclic olefin-catalyzed formation of cyclic carbonate from CO2 and propargylic alcohols were investigated by DFT calculations. Six mechanisms, four for the formation of five-membered cyclic carbonate (M-A, M-B, M-B’ and M-C), and two for six-membered cyclic carbonate (M-D and M-E), were fully investigated. The energy profiles in dichloromethane showed that M-B is the predominant reaction with the lowest barrier of 31.99 kcal mol−1, while M-C and M-D may be kinetically competitive to M-B. The very high activation energy of 45.37 kcal mol-1, 57.07 kcal mol-1 and 59.61 kcal mol−1 for M-A, M-B’ and M-E, respectively, suggest that they are of lesser importance in the overall mechanism.

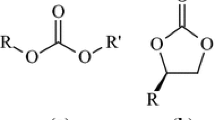
Formations of five-membered ring product and six-membered ring product are kinetically competitive, but five-membered ring product is thermodynamically more preferable.




Similar content being viewed by others
Notes
Note that intermediate 9 may take place via another reaction in which the H atom on the O1 atom migrates to the C3 atom, and the whole molecule decomposes to allyene, acetone and CO2. The barrier height for this reaction is 51.27 kcal mol−1 (relative to initial reactants)
References
Sakakura T, Choi J-C, Yasuda H (2007) Transformation of carbon dioxide. Chem Rev 107:2365–2387
Heldebrant DJ, Jessop PG, Thomas CA, Eckert CA, Liotta CL (2005) The reaction of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) with carbon dioxide. J Org Chem 70:5335–5338
Darensbourg DJ (2010) Chemistry of carbon dioxide relevant to its utilization: a personal perspective. Inorg Chem 49:10765–10780
Chen X-Y, Zhao Y-X, Wang S-G (2006) Relativistic DFT study on the reaction mechanism of second-row transition metal Ru with CO2. J Phys Chem A 110:3552–3558
Castro L, Lam OP, Bart SC, Meyer K, Maron L (2010) Carbonate formation from CO2 via oxo versus oxalate pathway: theoretical investigations into the mechanism of uranium-mediated carbonate formation. Organometallics 29:5504–5510
Li J, Lin Z (2009) Density functional theory studies on the reduction of CO2 to CO by a (NHC)Ni0 complex. Organometallics 28:4231–4234
Guo C-H, Song J-Y, Jia J-F, Zhang X-M, Wu H-S (2010) A DFTstudy on the mechanism of the coupling reaction between chloromethyloxirane and carbon dioxide catalyzed by Re(CO)5Br. Organometallics 29:2069–2079
Darensbourg DJ, Holtcamp MW (1996) Catalysts for the reactions of epoxides and carbon dioxide. Coord Chem Rev 153:155–174
Huang K, Sun CL, Shi ZJ (2011) Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem Soc Rev 40:2435–2452
Kleeberg C, Cheung MS, Lin Z, Marder TB (2011) Copper-mediated reduction of CO2 with pinB-SiMe2Ph via CO2 insertion into a copper-silicon bond. J Am Chem Soc 133:19060–19063
Boone MP, Stephan DW (2014) Ancillary metal centers in frustrated lewis pair chemistry: ruthenium acetylide as a Lewis base in the activation of CO2, aldehyde, and alkyne. Organometallics 33:387–393
Wen M, Huang F, Lu G, Wang ZX (2013) Density functional theory mechanistic study of the reduction of CO2 to CH4 catalyzed by an ammonium hydridoborate ion pair: CO2 activation via formation of a formic acid entity. Inorg Chem 52:12098–120107
Krogman JP, Foxman BM, Thomas CM (2011) Activation of CO2 by a heterobimetallic Zr/Co complex. J Am Chem Soc 133:14582–14585
Huang F, Zhang C, Jiang J, Wang ZX, Guan H (2011) How does the nickel pincer complex catalyze the conversion of CO2 to a methanol derivative? A computational mechanistic study. Inorg Chem 50:3816–3825
Ariafard A, Brookes NJ, Stranger R, Yates BF (2011) DFT study on the mechanism of the activation and cleavage of CO2 by (NHC)CuEPh3(E = Si, Ge, Sn). Organometallics 30:1340–1349
Jiang Y, Blacque O, Fox T, Berke H (2013) Catalytic CO2 activation assisted by rhenium hydride/B(C6F5)3 frustrated Lewis pairs—metal hydrides functioning as FLP bases. J Am Chem Soc 135:7751–7760
Barbarini A, Maggi R, Mazzacani A, Mori G, Sartori G, Sartorio R (2003) Cycloaddition of CO2 to epoxides over both homogeneous and silica-supported guanidine catalysts. Tetrahedron Lett 44:2931–2934
Carpenter BK (2007) Computational study of CO2 reduction by amines. J Phys Chem A 111:3719–3726
Gu Y, Shi F, Deng Y (2004) Ionic liquid as an efficient promoting medium for fixation of CO2: clean synthesis of r-methylene cyclic carbonates from CO2 and propargyl alcohols catalyzed by metal salts under mild conditions. J Org Chem 69:391–394
Gu Y, Zhang Q, Duan Z, Zhang J, Zhang S, Deng Y (2005) Ionic liquid as an efficient promoting medium for fixation of carbon dioxide: a clean method for the synthesis of 5-methylene-1,3-oxazolidin-2-ones from propargylic alcohols, amines, and carbon dioxide catalyzed by Cu(I) under mild conditions. J Org Chem 70:7376–7380
Sugawara Y, Yamada W, Yoshida S, Ikeno T, Yamada T (2007) Carbon dioxide-mediated catalytic rearrangement of propargyl alcohols into, α, β-unsaturated ketones. J Am Chem Soc 129:12902–12903
Kayaki Y, Yamamoto M, Ikariya T (2007) Stereoselective formation of r-alkylidene cyclic carbonates via carboxylative cyclization of propargyl alcohols in supercritical carbon dioxide. J Org Chem 72:647–649
Scondo A, Dumarcay F, Marsura A, Barth D (2010) Tandem Staudinger-Aza-Wittig reaction in supercritical CO2: synthesis of a pharmaceutical interest compound. J Supercrit Fluids 53:60–63
Lu X-B, Wang Y-B, Wang Y-M, Zhang W-Z (2013) Fast CO2 sequestration, activation, and catalytic transformation using N-heterocyclic olefin. J Am Chem Soc 135:11996–12003
Miertusˇ S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem Phys 55:117–129
Pascual-Ahuir JL, Silla E, Tomasi J, Bonaccorsi R (1987) Electrostatic interaction of a solute with a continuum. Improved description of the cavity and of the surface cavity bound charge distribution. J Comput Chem 8:778–787
Floris F, Tomasi J (1989) Evaluation of the dispersion contribution to the solvation energy. A simple computational model in the continuum approximation. J Comput Chem 10:616–627
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Frisch MJ (2010) Gaussian 03, Revision E.01. Gaussian, Inc, Wallingford
Frisch MJ (2004) Gaussian 09, Revision C.01. Gaussian, Inc, Wallingford
Lee C, Yang W, Parr RG (1988) Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Johnson ER, Mackie ID, DiLabio GA (2009) Dispersion interactions in density-functional theory. J Phys Org Chem 22:1127–1135
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–154119
Fromm A, van Wullen C, Hackenberger D, Goossen LJ (2014) Mechanism of Cu/Pd-catalyzed decarboxylative cross-couplings: a DFT investigation. J Am Chem Soc 136:10007–10023
Carpenter JE, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. THEOCHEM 46:41–62
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Acknowledgments
This project is supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Shanxi Province, P. R. China. Project No. 2008–75.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 3626 kb)
Rights and permissions
About this article
Cite this article
Yan, ZE., Huo, RP., Guo, Lh. et al. The mechanisms for N-heterocyclic olefin-catalyzed formation of cyclic carbonate from CO2 and propargylic alcohols. J Mol Model 22, 94 (2016). https://doi.org/10.1007/s00894-016-2959-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-2959-3