Abstract
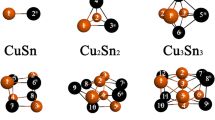
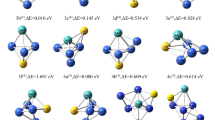
Neutral copper clusters are characterized through their molecular structures, binding energy and electric dipole polarizability. It is shown that the mean and anisotropy of polarizability tensor are useful properties in the characterization and rationalization of reactivity and growth patterns in copper clusters. We also found a relationship between softness per atom and cubic root polarizability per atom which can be useful to get global softness in copper clusters.






Similar content being viewed by others
References
Morse MD (1986) Chem Rev 86:1049
Spasov VA, Lee T-H, Ervin KM (2000) J Chem Phys 112:1713
Ingólfsson O, Busolt U, Sugawara K (2000) J Chem Phys 112:4613
Moore CE (1971) Atomic Energy Levels Vol. II of Nat Bur Standars
James AM, Lemire GW, Langridge-Smith PR (1994) Chem Phys Lett 277:503
Knickelbein MB (1992) Chem Phys Lett 192:129
Powers DE, Hansen SG, Geusic ME, Michalopoulos DL, Smalley RE (1983) J Chem Phys 78:2866
Winter BJ, Parks EK, Riley SJ (1991) J Chem Phys 92:8618
Ho J, Ervin KM, Lineberger WC (1990) J Chem Phys 93:6987
Balbuena P, Derosa P, Seminario JM (1999) J Phys Chem B 103:2830
Jug K, Zimmermann B, Calaminici P, Koster AM (2002) J Chem Phys 116:4497
Calaminici P, Koster AM, Vela A (2000) J Chem Phys 113:2199
Jackson KA (1993) Phys Rev B 47:9715
Chandrakumar KRS, Ghanty TK, Ghosh SK (2004) J Phys Chem A 108:6661
Jaque P, Toro-Labbé A (2002) J Chem Phys 117:3208
Jaque P, Toro-Labbé A (2004) J Phys Chem B 108:2568
Poater A, Duran M, Jaque P, Toro-Labbé A, Solà M (2006) J Phys Chem B 110:6526
Grigoryan VG, Alamanova D, Springborg M (2006) Phys Rev B 73:115415
Chu X, Xiang M, Zeng Q, Zhu W, Yang M (2011) J Phys B: At Mol Opt Phys 44:205103
Lecoultre S, Rydlo A, Fulix C, Buttet J, Gilb S, Harbich W (2011) J Chem Phys 134:074303
Chu X, Yang M, Jackson KA (2011) J Chem Phys 134:234505
Parka YH, Hijazib IA (2012) Mol Simul 38:241
Brack M (1993) Rev Mod Phys 65:677
De Heer W (1993) Rev Mod Phys 65:611
Bonin KD, Kresin VV
Knickelbein MB (2004) J Chem Phys 120:10450
Yang M, Jackson KA (2005) vol 122, pp 184317–1
Becke AD (1993) J Chem Phys 98:5648
Perdew JP, Wang WR (1992) Phys Rev B 45:13244
Hay PJ, Wadt WR (1985) J Chem Phys 82:270
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17:49
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Frisch JM, Trucks GW, Schlegel HB, et al (2003) Gaussian03. Revision C.02. Gaussian Inc. Pittsburgh, PA
Sadlej AJ (1988) Collect Czech Chem Commun 53:1995
Jaque P, Toro-Labbé A, Politzer P, Geerlings P (2008) Chem Phys Lett 456:135
Kittel C (1971) Introduction to Solid–State Physics. Wiley, New York, 4th edition
Pou-Amérigo R, Merchán M, Nebot-Gil I, Widmark PO, Ross BO (1995) Theor Chem Acc 92:149
Neogrady P, Killo V, Urban M, Sadlej AJ (1997) Int J Quantum Chem 63:557
Bishop DM, Kirtman B (1991) J Chem Phys 95:2646
Bishop DM, Kirtman B (1992) J Chem Phys 97:5255
Hirschfelder JO, Curtiss CF, Bird RB (1954) Molecular Theory of Gases and Liquids, page 966. Wiley, New York
Glasstone S (1940) Textbook of Physical Chemistry. van Nostrand, New York
Politzer P, Jin P, Murray JS (2002). J Chem Phys 117:8197
Millefiori S, Alparone A (2001). J Phys Chem A 105:9489
Aray Y, Rodriguez J, Vega D (2000) J Phys Chem B 104:4608
Parr RG, Yang W (1989) Density Functional Theory of Atoms and Molecules. Oxford University Press, New York
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Politzer P (1987) J Chem Phys 86:1072
Sen KD, Bohm MC, Schmidt PC (1987) Struct Bonding (Berlin) 66:99
Vela A, Gázquez JL (1990) J Am Chem Soc 112:1490
Fuentealba P, Reyes O (1993) J Mol Struct THEOCHEM 282:65
Ghanty TK, Ghosh SK (1993) J Phys Chem 97:4951
Simón-Manso Y, Fuentealba P (1998) J Phys Chem A 102:2029
Politzer P, Murray JS, Concha Monica C, Jin P (2007) Collect Czech Chem Commun 1:51
Yang W, Lee C, Ghosh SK (1985) J Phys Chem 89:5412
Acknowledgments
The authors acknowledge the financial support to FONDECYT through project numbers 1100291 and 1130072, and Millenium Nucleus CPC grant NC120082.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaque, P., Toro–Labbé, A. Polarizability of neutral copper clusters. J Mol Model 20, 2410 (2014). https://doi.org/10.1007/s00894-014-2410-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2410-6