Abstract
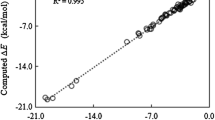
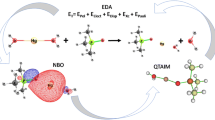
An assessment study is presented about energy decomposition analysis (EDA) in combination with DFT including revised dispersion correction (DFT-D3) with Slater-type orbital (STO) basis set. There has been little knowledge about the performance of the EDA + DFT-D3 concerning STOs. In this assessment such an approach was applied to calculate noncovalent interaction energies and their corresponding components. Complexes in S22 set were used to evaluate the performance of EDA in conjunction with four representative types of GGA-functionals of DFT-D3 (BP86-D3, BLYP-D3, PBE-D3 and SSB-D3) with three STO basis sets ranging in complexity from DZP, TZ2P to QZ4P. The results showed that the approach of EDA + BLYP-D3/TZ2P has a better performance not only in terms of calculating noncovalent interaction energy quantitatively but also in analyzing corresponding energy components qualitatively. This approach (EDA + BLYP-D3/TZ2P) was thus applied further to two representative large-system complexes including porphine dimers and fullerene aggregates to gain a better insight into binding characteristics.

The assessment of EDA+DFT-D3/STOs to noncovalent interactions






Similar content being viewed by others
References
Prins LJ, Scrimin P (2009) Angew Chem Int Ed 48:2288–2306
Dunitz JD, Gavezzotti A (2009) Chem Soc Rev 38:2622–2633
Černý J, Hobza P (2007) Phys Chem Chem Phys 9:5291–5303
Lein M, Frenking G (2005) The nature of the chemical bond in the light of an energy decomposition analysis. In: Dykstra CF, Frenking G, Kim KS, Scuseria GE (eds) Theory and applications of computational chemistry: the first forty years. Elsevier, Amsterdam, pp 291–372
Hopffgarten MV, Frenking G (2012) WIREs Comput Mol Sci 2:43–62
Mo Y, Bao P, Gao J (2011) Phys Chem Chem Phys 13:6760–6775
Su P, Li H (2009) J Chem Phys 131:014102
Reinhardt P, Piquemal JP, Savin A (2008) J Chem Theor Comput 4:2020–2029
Mitoraj MP, Michalak A, Ziegler T (2009) J Chem Theor Comput 5:962–975
Vyboishchikov SF, Krapp A, Frenking G (2008) J Chem Phys 129:144111
Cybulski H, Sadlej J (2008) J Chem Theor Comput 4:892–897
Rajchel Ł, Zuchowski PS, Szczesniak MM, Chałasinski G (2010) Phys Rev Lett 104:163001
Szalewicz K (2012) WIREs Comput Mol Sci 2:254–272
Jeziorski B, Moszynski R, Szalewicz K (1994) Chem Rev 94:1887–1930
Hohenstein EG, Sherrill CD (2010) J Chem Phys 133:014101
Morokuma K (1971) J Chem Phys 55:1236–1244
Morokuma K (1977) Acc Chem Res 10:294–300
Ziegler T, Rauk A (1977) Theor Chim Acta 46:1–10
Hesselmann A (2012) J Phys Chem A 115:11321–11330
Jurečka P, Šponer J, Černý J, Hobza P (2006) Phys Chem Chem Phys 8:1985–1993
Pitoňák M, Neogrády P, Černý J, Grimme S, Hobza P (2009) Chem Phys Chem 10:282–289
Marshall MS, Sears JS, Burns LA, Brédas JL, Sherrill CD (2010) J Chem Theory Comput 6:3681–3687
Pitoňák M, Řezáč J, Hobza P (2010) Phys Chem Chem Phys 12:9611–9614
Riley KE, Pitoňák M, Jurečka P, Hobza P (2010) Chem Rev 110:5023–5063
Pavlov A, Mitrasinovic PM (2010) Curr Org Chem 14:129–137
Sherrill CD (2009) Computations of Noncovalent π Interactionsin In: Lipkowitz KB, Cundari TR (Eds) Reviews in computational chemistry, vol 26, Wiley, New York, pp 1-38
Zhao Y, Schultz NE, Truhlar DG (2006) J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Grimme S (2006) J Comp Chem 27:1787–1799
von Lilienfeld OA, Tavernelli I, Rothlisberger U (2004) Phys Rev Lett 93:153004
Grimme S (2006) J Chem Phys 124:034108
Grimme S (2011) WIREs Comput Mol Sci 1:211–228
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
dftd3 program, see http://toc.uni-muenster.de/DFTD3/
Takatani T, Hohenstein EG, Malagoli M, Marshall MS, Sherrill CD (2010) J Chem Phys 132:144104
Riley KE, Pitoňák M, Černý J, Hobza P (2010) J Chem Theory Comput 6:66–80
van Lenthe E, Baerends EJ (2003) J Comput Chem 24:1142–1156
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305
Güell M, Luis JM, Solà M, Swart M (2008) J Phys Chem A 112:6384–6391
Burns LA, Vázquez-Mayagoitia Á, Sumpter BG, Sherrill CD (2011) J Chem Phys 134:084107
Grimme S, Antony J, Schwabe T, Mück-Lichtenfeld C (2007) Org Biomol Chem 5:741–758
Goerigk L, Grimme S (2011) Phys Chem Chem Phys 13:6670–6688
Peverati R, Baldridge KK (2008) J Chem Theory Comput 4:2030–2048
Thanthiriwatte KS, Hohenstein EG, Burns LA, Sherrill CD (2011) J Chem Theory Comput 7:88–96
Hohenstein EG, Chill ST, Sherrill CD (2008) J Chem Theory Comput 4:1996–2000
Korth M, Thiel W (2011) J Chem Theory Comput 7:2929–2936
Goerigk L, Grimme S (2010) J Chem Theory Comput 6:107–126
Safonov AA, Rykova EA, Bagaturyants AA, Sazhnikov VA, Alfimov MV (2011) J Mol Model 17:1855–1862
Mück-Lichtenfeld C, Grimme S (2007) Mol Phys 105:2793–2798
Baerends EJ, Autschbach J, Bashford D, Bérces A, Bickelhaupt FM, Bo C, Boerrigter PM, Cavallo L, Chong DP, Deng L, Dickson RM, Ellis DE, van Faassen M, Fan L, Fischer TH, Fonseca Guerra C, Ghysels A, Giammona A, van Gisbergen SJA, Götz AW, Groeneveld JA, Gritsenko OV, Grüning M, Harris FE, van den Hoek P, Jacob CR, Jacobsen H, Jensen L, van Kessel G, Kootstra F, Krykunov MV, van Lenthe E, McCormack DA, Michalak A, Mitoraj M, Neugebauer J, Nicu VP, Noodleman L, Osinga VP, Patchkovskii S, Philipsen PHT, Post D, Pye CC, Ravenek W, Rodríguez JI, Ros P, Schipper PRT, Schreckenbach G, Seth M, Snijders JG, Solà M, Swart M, Swerhone D, te Velde G, Vernooijs P, Versluis L, Visscher L, Visser O, Wang F, Wesolowski TA, van Wezenbeek EM, Wiesenekker G, Wolff SK, Woo TK, Yakovlev AL, Ziegler T (2012) ADF version 2012.01, SCM, Theoretical Chemistry. Vrije Universiteit, Amsterdam, http://www.scm.com
Grimme S, Ehrlich S, Goerigk L (2011) J Comput Chem 32:1456–1465
Riley KE, Hobza P (2008) J Chem Theory Comput 4:232–242
Ohira S, Brédas J-L (2009) J Mater Chem 19:7545–7550
Ahn TK, Kim KS, Kim DY, Noh SB, Aratani N, Ikeda C, Osuka A, Kim D (2006) J Am Chem Soc 128:1700–1704
Aittala PJ, Cramariuc O, Hukka TI (2011) Chem Phys Lett 501:226–231
Day PN, Nguyen KA, Pachter R (2008) J Chem Theory Comput 4:1094–1106
Yang Q-Z, Khvostichenko D, Atkinson JD, Boulatov R (2008) Chem Commun 38:963–965
Podeszwa R, Szalewicz K (2008) Phys Chem Chem Phys 10:2735–2746
Hohenstein EG, Sherrill CD (2009) J Phys Chem A 113:878–886
Gayathri SS, Wielopolski M, Pérez EM, Fernández G, Sánchez L, Viruela R, Ortí E, Guldi DM, Martín N (2009) Angew Chem Int Ed 48:815–819
González-Rodríguez D, Carbonell E, Guldi DM, Torres T (2009) Angew Chem Int Ed 48:8032–8036
Pérez EM, Capodilupo AL, Fernández G, Sánchez L, Viruela PM, Viruela R, Ortí E, Bietti M, Martín N (2008) Chem Commun 38:4567–4569
Pérez EM, Martín N (2008) Chem Soc Rev 37:1512–1519
Kawase T, Kurata H (2006) Chem Rev 106:5250–5273
Wong BM (2009) J Comput Chem 30:51–56
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09 C01. Gaussian Inc, Wallingford, CT
Acknowledgments
Financial support by the National Natural Science Foundation of China (No. 21173069) is acknowledged. We are grateful to Dr. Andreas Hesselmann at Lehrstuhl für Theoretische Chemie, Universität Erlangen-Nürnberg for his results of SAPT-DFT calculation. Authors would also like to thank Prof. Christian Mück-lichtenfeld at Organisch-Chemisches Institut der Universität Münster for his structure of porphine dimers. The computation of the Gaussian is supported by the School of Chemical and Environmental Sciences, Henan Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, W., Feng, H., Xuan, X. et al. The assessment and application of an approach to noncovalent interactions: the energy decomposition analysis (EDA) in combination with DFT of revised dispersion correction (DFT-D3) with Slater-type orbital (STO) basis set. J Mol Model 18, 4577–4589 (2012). https://doi.org/10.1007/s00894-012-1425-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1425-0