Abstract
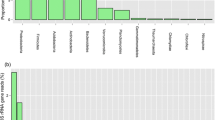
Three 16S rRNA gene clone libraries (P1L, P4L and P8L) were constructed using three soil samples (P1S, P4S and P8S) collected near Pindari glacier, Himalayas. The three libraries yielded a total of 703 clones. Actinobacteria, Firmicutes and Proteobacteria were common to the three libraries. In addition to the above P1L and P8L shared the phyla Acidobacteria, Bacteroidetes, Gemmatimonadetes and Planctomycetes. Phyla Chlamydiae, Chlorobi, Chloroflexi, Dictyoglomi, Fibrobacteres, Nitrospirae, Verrucomicrobia, candidate division SPAM and candidate TM7s TM7a phylum were present only in P1L. Rarefaction analysis indicated that the bacterial diversity in P4S and P8S soil samples was representative of the sample. Principal component analysis (PCA) revealed that P1S and P8S were different from P4S soil sample. PCA also indicated that arsenic content, pH, Cr and altitude influence the observed differences in the percentage of specific OTUs in the three 16S rRNA gene clone libraries. The observed bacterial diversity was similar to that observed for other Himalayan and non-polar cold habitats. A total of 40 strains of bacteria were isolated from the above three soil samples and based on the morphology 20 bacterial strains were selected for further characterization. The 20 bacteria belonged to 12 different genera. All the isolates were psychro-, halo- and alkalitolerant. Amylase and urease activities were detected in majority of the strains but lipase and protease activities were not detected. Long chain, saturated, unsaturated and branched fatty acids were predominant in the psychrotolerant bacteria.












Similar content being viewed by others
References
Abyzov SS, Mitskevich IN, Poglazova MN (1998) Microflora of the deep glacier horizons of central Antarctica. Microbiology 67:66–73
Ageta Y, Kadota T (1992) Predictions of changes of glacier mass balance in the Nepal Himalaya and Tibetan Plateau: a case study of air temperature increase for three glaciers. Ann Glaciol 16:89–94
Blakemore LC, Searle PL, Daly BK (1987) Methods for chemical analysis of soils. NZ Soil Bureau Scientific Report 80
Booth C (1978) Introduction to general methods. In: Booth C (ed) Methods Microbiol. Academic Press, New York, pp 57–91
Brambilla E, Hippe H, Hagelstein A, Tindall BJ, Stackebrandt E (2001) 16S rDNA diversity of cultured and uncultured prokaryotes of a mat sample from Lake Fryxell, McMurdo Dry Valleys, Antarctica. Extremophiles 5:23–33
Brinkmeyer R, Knittel K, Jürgens J, Weyland H, Amann R, Helmke E (2003) Diversity and community structure of bacterial communities in Arctic versus Antarctic sea ice. Appl Environ Microbiol 69:6610–6619
Certini G, Campbell CD, Edwards AC (2004) Rock fragments in soil support a different microbial community from the fine earth. Soil Biol Biochem 36:1119–1128
Chaturvedi P, Shivaji S (2006) Exiguobacterium indicum sp. nov., a psychrophilic bacterium from the Hamta glacier of the Himalayan mountain ranges of India. Int J Syst Evol Microbiol 56:2765–2770
Chaturvedi P, Reddy GSN, Shivaji S (2005) Dyadobacter hamtensis sp. nov., from Hamta glacier, located in the Himalayas, India. Int J Syst Evol Microbiol 55:2113–2117
Cheng SM, Foght JM (2007) Cultivation-independent and -dependent characterization of bacteria resident beneath John Evans glacier. FEMS Microbiol Ecol 59:318–330
Chintalapati S, Kiran MD, Shivaji S (2004) Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol 50:631–642
Christner BC, Mosley-Thompson E, Thompson LG, Reeve JN (2001) Isolation of bacteria and 16S rDNAs from Lake Vostok accretion ice. Environ Microbiol 3:570–577
Christner BC, Mosley-Thompson E, Thompson LG, Reeve JN (2003) Bacterial recovery from ancient glacial ice. Environ Microbiol 5:433–436
Costello EK, Schmidt SK (2006) Microbial diversity in alpine tundra wet meadow soil: novel Chloroflexi from a cold, water-saturated environment. Environ Microbiol 8:1471–1486
Dancer SJ, Shears P, Platt DJ (1997) Isolation and characterization of coliforms from glacial ice and water in Canada’s High Arctic. J Appl Microbiol 82:597–609
Dong H, Zhang G, Jiang H, Yu B, Chapman LR, Lucas CR, Fields MW (2006) Microbial diversity in sediments of saline Qinghai Lake, China: linking geochemical controls to microbial ecology. Microbiol Ecol 51:65–82
Dyurgerov MB, Meier MF (1997) Mass balance of mountain and subpolar glaciers: a new global assessment for 1961–1990. Arct Alp Res 29:379–391
Eichorst SA, Breznak JA, Schmidt TM (2007) Isolation and characterization of soil bacteria that define Terriglobus gen. nov. in the phylum Acidobacteria. Appl Environ Microbiol 73:2708–2717
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1:200–208
Foght J, Aislabie J, Turner S, Brown CE, Ryburn J, Saul DJ, Lawson W (2004) Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers. Microbiol Ecol 47:329–340
Fushimi H, Ikegami K, Higuchi K, Shankar K (1985) Nepal case study: catastrophic floods. In: Young GJ (ed) Techniques for prediction of runoff from glacierized areas (publication 149). International Association of Hydrological Sciences, Wallingford, UK, pp 125–130
Gangwar P, Alam SI, Bansod S, Singh L (2009) Bacterial diversity of soil samples from the western Himalayas, India. Can J Microbiol 55:564–577
Gomes J, Steiner W (2004) The biocatalytic potential of extremophiles and extremozymes. Food Technol Biotechnol 42:223–235
Good IJ (1954) The population frequencies of species and the estimation of population parameters. Biometrica 40:237–264
Hur I, Chun J (2004) A method for comparing multiple bacterial community structures from 16S rDNA clone library sequences. J Microbiol 42:9–13
Jackson ML (1967) Soil chemical analysis. Prentice–Hall of India, New Delhi
Kastovská K, Elster J, Stibal M, Santrůcková H (2005) Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (High Arctic). Microbiol Ecol 50:396–407
Kishore KH, Begum Z, Pathan AA, Shivaji S (2010) Paenibacillus glacialis sp. nov., isolated from Kafni glacier of Himalayas, India. Int J Syst Evol Microbiol (in press)
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–147
Lefauconnier B, Hagen JO, Rudant JP (1994) Flow speed and calving rate of Kongsbreen glacier, Svalbard, using SPOT images. Pol Res 10:56–65
Li Y, Li F, Zhang X, Qin S, Zeng Z, Dang H, Qin Y (2008) Vertical distribution of bacterial and archaeal communities along discrete layers of a deep-sea cold sediment sample at the East Pacific Rise (approximately 13 degrees N). Extremophiles 12:573–585
Lipson DA, Schmidt SK (2004) Seasonal changes in an alpine soil bacterial community in the Colorado Rocky Mountains. Appl Environ Microbiol 70:2867–2879
Liu Y, Yao T, Jiao N, Kang S, Zeng Y, Huang S (2006) Microbial community structure in moraine lakes and glacial meltwaters, Mount Everest. FEMS Microbiol Lett 265:98–105
Liu Y, Yao T, Jiao N, Kang S, Xu B, Zeng Y, Huang S, Liu X (2009) Bacterial diversity in the snow over Tibetan Plateau Glaciers. Extremophiles 13:411–423
Lysnes K, Thorseth IH, Steinsbu BO, Øvreås L, Torsvik T, Pedersen RB (2004) Microbial community diversity in seafloor basalt from the Arctic spreading ridges. FEMS Microbiol Ecol 50:213–230
Magurran AE (1996) (ed) Ecological diversity and its measurement. Chapman–Hall, London, pp 274–286
Marcy Y, Ouverney C, Bik EM, Losekann T, Ivanova N, Martin HG, Szeto E, Platt D, Hugenholtz P, Relman DA, Quake SR (2007) Dissecting biological ‘dark matter’ with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc Natl Acad Sci USA 104:11889–11894
Mayilraj S, Prasad GS, Suresh K, Saini HS, Shivaji S, Chakrabarti T (2005) Planococcus stackebrandtii sp. nov., isolated from a cold desert of the Himalayas, India. Int J Syst Evol Microbiol 55:91–94
Mindl B, Anesio AM, Meirer K, Hodson AJ, Laybourn-Parry J, Sommaruga R, Sattler B (2007) Factors influencing bacterial dynamics along a transect from supraglacial runoff to proglacial lakes of a high Arctic glacier. FEMS Microbiol Ecol 59:307–317
Miteva VI, Sheridan PP, Brenchley JE (2004) Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core. Appl Environ Microbiol 70:202–213
Mosier AC, Murray AE, Fritsen CH (2007) Microbiota within the perennial ice cover of Lake Vida, Antarctica. FEMS Microbiol Ecol 59:274–288
Nemergut DR, Anderson SP, Cleveland CC, Martin AP, Miller AE, Seimon A, Schmidt SK (2007) Microbial community succession in an unvegetated, recently deglaciated soil. Microbiol Ecol 53:110–122
Nishida I, Murata N (1996) Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol 47:541–568
Pindi PK, Kishore HK, Reddy GSN, Shivaji S (2009) Description of Leifsonia kafniensis sp. nov. and Leifsonia antarctica sp. nov. Int J Syst Evol Microbiol 59:1348–1352
Pradhan S, Srinivas TNR, Pindi PK, Kishore KH, Begum Z, Singh PK, Singh AK, Pratibha MS, Yasala AK, Reddy GS, Shivaji S (2010) Bacterial biodiversity from Roopkund glacier, Himalayan mountain ranges, India. Extremophiles 14:377–395
Priest FG (1977) Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev 41:711–753
Priscu JC, Christner BC (2003) Microbial diversity and bioprocessing. In: Bull AT (ed) Earth’s icy biosphere. ASM Press, Washington DC, pp 130–145
Reddy GSN, Aggarwal RK, Matsumotto GI, Shivaji S (2000) Arthrobacter flavus sp. nov., a psychrophilic bacterium isolate from a pond in McMurdo Dry Valley, Antarctica. Int J Syst Evol Microbiol 50:1553–1561
Reddy GSN, Prabagaran SR, Shivaji S (2008a) Leifsonia pindariensis sp. nov., isolated from the Pindari glacier of the Indian Himalayas, and emended description of the genus Leifsonia. Int J Syst Evol Microbiol 58:2229–2234
Reddy GSN, Uttam A, Shivaji S (2008b) Bacillus cecembensis sp. nov., isolated from the Pindari glacier of the Indian Himalayas. Int J Syst Evol Microbiol 58:2330–2335
Reddy GSN, Pradhan S, Manorama R, Shivaji S (2010) Cryobacterium Pindariense sp. nov., a psychrophilic bacterium from a Himalayan glacier. Int J Syst Evol Microbiol 60:866–870
Reed AJ, Lutz RA, Vetriani C (2006) Vertical distribution and diversity of bacteria and archaea in sulfide and methane-rich cold seep sediments located at the base of the Florida Escarpment. Extremophiles 10:199–211
Rhoades JD (1982) Soluble salts. In: Page AL (ed) Methods of soil analysis. Part 2, 2nd edn. Agronomy monograph No. 9. American Society of Agronomy, Madison, WI, pp 167–179
Russell NJ (1998) Molecular adaptations in psychrophilic bacteria: potential for biotechnological applications. Adv Biochem Eng Biotechnol 61:1–21
Segawa T, Miyamoto K, Ushida K, Agata K, Okada N, Kohshima S (2005) Seasonal change in bacterial flora and biomass in mountain snow from the Tateyama Mountains, Japan, analyzed by 16S rRNA gene sequencing and real-time PCR. Appl Environ Microbiol 71:123–130
Shivaji S, Shyamala Rao N, Saisree L, Sheth V, Reddy GSN, Bhargava PM (1989) Isolation and identification of Pseudomonas species from Schirmacher Oasis, Antarctica. Appl Environ Microbiol 55:767–771
Shivaji S, Reddy GSN, Aduri RP, Kutty R, Ravenschlag K (2004) Bacterial diversity of a soil sample from Schirmacher Oasis, Antarctica. Cell Mol Biol 50:525–536
Shivaji S, Kiran MD, Chintalapati S (2007) Perception and transduction of low temperature in bacteria. In: Gerday C, Glansdorff N (eds) Physiology and biochemistry of extremophiles. ASM Press, Washington, DC, pp 194–207
Skidmore M, Anderson SP, Sharp M, Foght J, Lanoil BD (2005) Comparison of microbial community composition in two subglacial environments reveals a possible role for microbes in chemical weathering processes. Appl Environ Microbiol 71:6986–6997
Srinivas TNR, Nageswara Rao SSS, Vishnu Vardhan Reddy P, Pratibha MS, Sailaja B, Kavya B, Hara Kishore K, Begum Z, Singh SM, Shivaji S (2009) Bacterial diversity and bioprospecting for cold-active lipases, amylases and proteases, from culturable bacteria of Kongsfjorden and Ny-Ålesund, Svalbard, Arctic. Curr Microbiol 59:537–547
Steven B, Briggs G, McKay CP, Pollard WH, Greer CW, Whyte LG (2007) Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture- dependent and culture-independent methods. FEMS Microbiol Ecol 59:513–523
Treves DS, Xia B, Tiedje JM (2003) A two-species test of the hypothesis that spatial isolation influences microbial diversity in soil. Microbiol Ecol 45:20–28
Tsai YL, Olson BH (1991) Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol 57:1070–1074
Tsai YL, Olson BH (1992) Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol 58:2292–2295
Vardhan Reddy VP, Nageswara SSS, Pratibha MS, Sailaja B, Kavya B, Ruth Manorama R, Singh SM, Srinivas TNR, Shivaji S (2009) Bacterial diversity and bioprospecting for cold-active enzymes from culturable bacteria associated with sediment of melt water stream of Midtre Lov′enbreen glacier, an Arctic glacier. Res Microbiol 160:538–546
Wagner D, Kobabe S, Liebner S (2009) Bacterial community structure and carbon turnover in permafrost-affected soils of the Lena Delta, northeastern Siberia. Can J Microbiol 55:77–83
Xiang SR, Yao TD, An LZ, Xu BQ, Li Z, Wu GJ, Wang YQ, Ma S, Chen XR (2004) Bacterial diversity in Malan ice core from the Tibetan Plateau. Folia Microbiol 49:269–275
Xiang S, Yao T, An L, Xu B, Wang J (2005) 16S rRNA sequences and differences in bacteria isolated from the Muztag Ata glacier at increasing depths. Appl Environ Microbiol 71:4619–4627
Xiang SR, Yao TD, Wu GJ, Chen Y, Shang TC, Pu LL, An LZ (2006) Deposition properties of bacterial populations in the Muztag Ata ice core. Quat Sci 26:185–191
Xiang SR, Shang TC, Chen Y, Jing ZF, Yao T (2009) Dominant bacteria and biomass in the Kuytun 51 glacier. Appl Environ Microbiol 75:7287–7290
Yamada T (1991) Preliminary report on glacier lake outburst flood in the Nepal Himalayas, Report 4/1/291191/1/l, Seq. No. 387. Water and Energy secretariat, HMG, Kathmandu, Nepal
Yamada T, Shiraiwa T, Iida H, Kadota T, Watanabe T, Rana B, Ageta Y, Fushimi H (1992) Fluctuations of the glaciers from the 1970s to 1989 in the Khumbu, Shorong and Langtang regions, Nepal Himalayas. Bull Glaciol Res 10:11–19
Zhang X, Tandong Y, Lizhe A, Lide T, Shijian X (2006) A study on the vertical profile of bacterial DNA structure in the Puruogangri (Tibetan Plateau) ice core using denaturing gradient gel electrophoresis. Ann Glaciol 43:160–166
Zhang S, Hou S, Ma X, Qin D, Chen T (2007) Culturable bacteria in Himalayan glacial ice in response to atmospheric circulation. Biogeoscience 4:1–9
Zhang X, Yao T, Tian L, Xu S, An L (2008) Phylogenetic and physiological diversity of bacteria isolated from Puruogangri ice core. Microbiol Ecol 55:476–488
Zhou J, Davey ME, Figueras JB, Rivkina E, Gilichinsky D, Tiedje JM (1997) Phylogenetic diversity of a bacterial community determined from Siberian tundra soil DNA. Microbiol 143:3913–3919
Acknowledgments
We would like to thank the Department of Biotechnology and Council of Scientific and Industrial Research, Government of India, for financial support to Dr. S. Shivaji. T.N.R.S. acknowledges the CSIR, Government of India, for the award of Research Associateship. We would like to thank Dr. S.M. Singh, Scientist, from National Centre for Antarctic Ocean Research, Goa, for providing the data on the soil chemical characteristics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Matsunaga.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shivaji, S., Pratibha, M.S., Sailaja, B. et al. Bacterial diversity of soil in the vicinity of Pindari glacier, Himalayan mountain ranges, India, using culturable bacteria and soil 16S rRNA gene clones. Extremophiles 15, 1–22 (2011). https://doi.org/10.1007/s00792-010-0333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-010-0333-4