Abstract
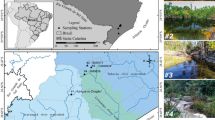
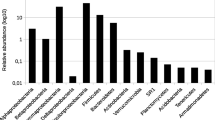
Mining of metallic sulfide ore produces acidic water with high metal concentrations that have harmful consequences for aquatic life. To understand the composition and structure of microbial communities in acid mine drainage (AMD) waters associated with Zn mine tailings, molecular diversity of 16S genes was examined using a PCR, cloning, and sequencing approach. A total of 78 operational taxonomic units (OTUs) were obtained from samples collected at five different sites in and around mining residues in Sepetiba Bay, Brazil. We analyzed metal concentration, physical, chemical, and microbiological parameters related to prokaryotic diversity in low metal impacted compared to highly polluted environments with Zn at level of gram per liter and Cd–Pb at level of microgram per liter. Application of molecular methods for community structure analyses showed that Archaea and Bacteria groups present a phylogenetic relationship with uncultured environmental organisms. Phylogenetic analysis revealed that bacteria present at the five sites fell into seven known divisions, α-Proteobacteria (13.4%), β-Proteobacteria (16.3%), γ-Proteobacteria (4.3%), Sphingobacteriales (4.3%), Actinobacteria (3.2%) Acidobacteria (2.1%), Cyanobacteria (11.9%), and unclassified bacteria (44.5%). Almost all archaeal clones were related to uncultivated Crenarchaeota species, which were shared between high impacted and low impacted waters. Rarefaction curves showed that bacterial groups are more diverse than archaeal groups while the overall prokaryotic biodiversity is lower in high metal impacted environments than in less polluted habitats. Knowledge of this microbial community structure will help in understanding prokaryotic diversity, biogeography, and the role of microorganisms in zinc smelting AMD generation and perhaps it may be exploited for environmental remediation procedures in this area.




Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Amado Filho GM, Karez CS, Andrade LR, Yoneshigue-Valentin Y, Pfeiffer WC (1997) Effects on growth and accumulation of zinc in six seaweed species. Ecotoxicol Environ Saf 37:223–228
Amann RI, Stromley J, Devereux R, Key R, Stahl DA (1992) Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol 58:614–623
Andrade L, Gonzalez AM, Araújo FV, Paranhos R (2003) Flow cytometry assessment of bacterioplankton in tropical marine environments. J Microbiol Methods 55:841–850
Bond PL, Druschel GK, Banfield JF (2000) Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems. Appl Environ Microb 66:4962–4971
Brãuer SL, Cadillo-Quiroz H, Yashiro E, Yavitt JB, Zinder SH (2006) Isolation of a novel acidiphilic methanogen from an acid peat bog. Nature 442:192–194
Cánovas CR, Olías M, Nieto JM, Sarmiento AM, Cerón JC (2007) Hydrogeochemical characteristics of the Tinto and Odiel Rivers (SW Spain). Factors controlling metal contents. Sci Total Environ 373:363–382
Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, Chandra S, McGarrell DM, Schmidt TM, Garrity GM, Tiedje JM (2003) The ribosomal database project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res 31:442–443
Correa Junior JD, Allodi S, Amado-Filho GM, Farina M (2000) Zinc accumulation in phosphate granules of Ucides cordatus hepatopancreas. Braz J Med Biol Res 33:217–221
DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 12:5685–5689
Dopson M, Baker-Austin C, Koppineed PR, Bond PL (2003) Growth in sulfidic mineral environments: metal resistance mechanism in acidophilic microorganisms. Microbiol 149:1959–1970
Edwards KJ, Bond PL, Gihring TM, Banfield JF (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:1796–1799
Edwing B, Hillier L, Wendl M, Green P (1998) Base-calling of automated sequencer traces using phred accuracy assessment. Gen Res 8:175–185
Gasol JM, del Giorgio PA (2000) Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64:197–224
Giovannoni SJ, Stingl U (2005) Molecular diversity and ecology of microbial plankton. Nature 15:343–348
Grasshoff K, Kremling K, Erhardt M (1999) Methods of seawater analysis, 3rd edn. Wiley–VCH Verlag, Germany, p 600
Hallberg KB, Coupland K, Kimura S, Johnson DB (2006) Macroscopic streamer growths in acidic, metal-rich mine waters in north wales consist of novel and remarkably simple bacterial communities. Appl Environ Microbiol 72:2022–2030
He Z, Xiao S, Xie X, Zhong H, Hu Y, Li Q, Gao F, Li G, Liu J, Qiu G (2007) Molecular diversity of microbial community in acid mine drainages of Yunfu sulfide mine. Extremophiles 11:305–314
He Z, Xiao S, Xie X, Hu Y (2008) Microbial diversity in acid mineral bioleaching systems of dongxiang copper mine and Yinshan lead–zinc mine. Extremophiles 12:225–234
Heck KL, van Belle G, Simberloff D (1975) Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology 56:1459–1461
Hurlbert SH (1971) The nonconcept of species diversity: a critique and alternative parameters. Ecology 52:577–586
Johnson DB (1998) Biodiversity and ecology of acidophilic microorganisms. FEMS Microb Ecol 27:307–317
Johnson DB, Rolfe S, Hallberg KB, Iversen E (2001) Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine. Environ Microbiol 3:630–637
Johnson DB, Hallberg KB (2003) The microbiology of acidic mine waters. Res Microbiol 154:466–473
Junior RGSL, Araújo FG, Maia MF, Pinto ASSB (2002) Evaluation of heavy metals in fish of the Sepetiba and Ilha Grande Bays, Rio de Janeiro, Brazil. Environ Res 89:171–179
Kamjunke N, Tittel J, Krumbeck H, Beulker C, Poerschmann J (2005) High heterotrophic bacterial production in acidic, iron-rich mining lakes. Microb Ecol 49:425–433
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Lacerda LD, Pfeiffer WC, Fiszman M (1987) Heavy metal distribution, availability and fate in Sepetiba Bay, S.E. Brazil. Sci Total Environ 65:163–173
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lohr AJ, Laverman AM, Braster M, van Straalen NM, Roling WF (2006) Microbial communities in the world’s largest acidic volcanic lake, Kawah Ijen in Indonesia, and in the Banyupahit River originating from it. Microb Ecol 2:609–618
Maeda S, Sakaguchi T (1990) Accumulation and detoxification of toxic metal elements by algae. In: Akatsuka I (ed) Introduction to applied phycology. Acad Publish, The Netherlands, pp 109–136
Molisani MM, Martins RV, Machado W, Paraquetti HHM, Bidone ED, Lacerda LD (2004) Environmental changes in Sepetiba Bay, SE Brazil. Reg Environ Change 4:17–27
Nicomrat D, Dick WA, Tuovinen OH (2006) Assessment of the microbial community in a constructed wetland that receives acid coal mine drainage. Microb Ecol 51:83–89
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc Natl Acad Sci 96:3455–3468
Peplow D, Edmonds R (2005) The effects of mine waste contamination at multiple levels of biological organization. Ecol Eng 24:101–119
Rappe MS, Giovannoni SJ (2003) The uncultured microbial majority. Ann Rev Microbiol 57:369–394
Rühling A, Tyler G (1973) Heavy metal pollution and decomposition of spruce needle litter. Oikos 24:402–416
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
SEMADS (2001) Bacias Hidrográficas e Recursos Hídricos da Macroregião Ambiental 2 - Bacia da Baía de Sepetiba. Rio de Janeiro 1:79
Singleton DR, Furlong MA, Ratbhun SL, Whitman WB (2001) Quantitative comparisons of 16S rRNA Gene Sequence Libraries from Environmental Samples. Appl Environ Microbiol 67:4374–4376
Smith DC, Azam F (1992) A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-leucine. Mar Microb Food Webs 6:107–114
Somerville CC, Knight IT, Straube WL, Colwell RR (1989) Simple rapid method for direct isolation of nucleic acids from aquatic environments. Appl Environ Microbiol 55:548–554
Takai K, Moser DP, DeFlaun M, Onstott TC, Fredrickson JK (2001) Archaeal diversity in waters from deep South African gold mines. Appl Environ Microbiol 67:5750–5760
Tan GL, Shu WS, Hallberg KB, Li F, Lan CY, Huang LN (2006) Cultivation-dependent and cultivation-independent characterization of the microbial community in acid mine drainage associated with acidic Pb/Zn mine tailings at Lechang, Guangdong, China. FEMS Microbiol Ecol 59:118–126
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Tyler G (1974) Heavy metal pollution and soil enzymatic activity. Plant Soil 41:303–311
Tyson GW, Chapman J, Hugenholtz P, Allen EE, Ram RJ, Richardson PM, Solovyev VV, Rubin EM, Rokhsar DS, Banfield JF (2004) Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 4:37–43
Urbach E, Kevin LV, Young L, Morse A, Larson GL, Giovannoni SJ (2001) Unusual bacterioplankton community structure in ultra-oligotrophic Crater Lake. Limnol Oceanogr 46:557–572
Vieira RP, Clementino MM, Cardoso AM, Oliveira DN, Albano RM, Gonzalez AM, Paranhos R, Martins OB (2007) Archaeal communities in a tropical estuarine ecosystem: Guanabara Bay, Brazil. Microb Ecol 54:460–468
Wendt-Potthoff K, Koschorreck M (2002) Functional groups and activities of bacteria in a highly acidic volcanic mountain stream and lake in Patagonia, Argentina. Microb Ecol 43:92–106
Yin H, Cao L, Qiu G, Wang D, Kellog L, Zhou J, Liu X, Dai Z, Ding J, Liu X (2008) Molecular diversity of 16S rRNA and gyrB genes in copper mines. Arch Microbiol 189:101–110
Zang HB, Yang MX, Shi W, Zheng Y, Sha T, Zhao ZW (2007) Bacterial diversity in mine tailings compared by cultivation and cultivation-independent methods and their resistance to lead and cadmium. Microb Ecol 54:702–712
Acknowledgments
We acknowledge the Genome Sequencing facilities core Johanna Döbereiner at IBqM/UFRJ, the Limnology Laboratory of UFRJ for access to liquid scintillator. We are grateful to João Paulo M. Torres and Valéria Magalhães for manuscript review. This work was supported by Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
Rights and permissions
About this article
Cite this article
Almeida, W.I., Vieira, R.P., Cardoso, A.M. et al. Archaeal and bacterial communities of heavy metal contaminated acidic waters from zinc mine residues in Sepetiba Bay. Extremophiles 13, 263–271 (2009). https://doi.org/10.1007/s00792-008-0214-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-008-0214-2