Abstract
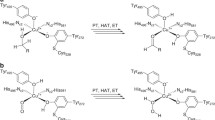
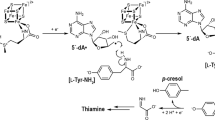
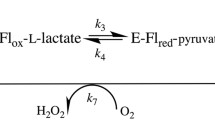
The catalytic mechanism of the copper-containing enzyme galactose oxidase involves a protein radical on Tyr272, one of the equatorial copper ligands. The first step in this mechanism has been proposed to be the abstraction of a proton from the alcohol substrate by Tyr495, the axial copper ligand that is weakly co-ordinated to copper. In this study we have generated and studied the properties of a Y495F variant to test this proposal. X-ray crystallography reveals essentially no change from wild-type other than loss of the tyrosyl hydroxyl group. Visible spectroscopy indicates a significant change in the oxidised Y495F compared to wild-type with loss of a broad 810-nm peak, supporting the suggestion that this feature is due to inter-ligand charge transfer via the copper. The presence of a peak at 420 nm indicates that the Y495F variant remains capable of radical formation, a fact supported by EPR measurements. Thus the significantly reduced catalytic efficiency (1100-fold lower k cat / K m) observed for this variant is not due to an inability to generate the Tyr272 radical. By studying azide-induced pH changes, it is clear that the reduced catalytic efficiency is due mainly to the inability of Y495F to accept protons. This provides definitive evidence for the key role of Tyr495 in the initial proton abstraction step of the galactose oxidase catalytic mechanism.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 17 December 1996 / Accepted: 12 March 1997
Rights and permissions
About this article
Cite this article
Reynolds, M., Baron, A., Wilmot, C. et al. Structure and mechanism of galactose oxidase: catalytic role of tyrosine 495. JBIC 2, 327–335 (1997). https://doi.org/10.1007/s007750050139
Issue Date:
DOI: https://doi.org/10.1007/s007750050139