Abstract
Introduction
Cortical thickness of the femoral diaphysis is assumed to be a preferred parameter in the assessment of the structural adaptation by mechanical use and biological factors. This study aimed to investigate the age- and sex-specific characteristics in cortical thickness of the femoral diaphysis between young and elderly non-obese people.
Materials and methods
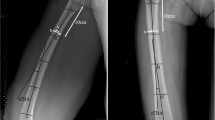
This study investigated 34 young subjects (21 men and 13 women; mean age: 27 ± 8 years) and 52 elderly subjects (29 men and 23 women; mean age: 70 ± 6 years). Three-dimensional (3D) cortical thickness of the femoral diaphysis was automatically calculated for 5000–8000 measurement points using the high-resolution cortical thickness measurement from clinical CT data. In 12 assessment regions created by combining three heights (proximal, central, and distal diaphysis) and four areas of the axial plane at 90° (medial, anterior, lateral, and posterior areas) in the femoral coordinate system, the standardized thickness was assessed using the femoral length.
Results
As per the trends, (1) there were no differences in medial and lateral thicknesses, while the posterior thickness was greater than the anterior thickness, (2) the thickness in men was higher than that in women, and (3) the thickness in young subjects was higher than that in elderly subjects.
Conclusions
The results of this study are of clinical relevance as reference points to clarify the causes of various pathological conditions for diseases of the lower extremities.



Similar content being viewed by others
References
Frost HM (2004) The Utah paradigm of skeletal physiology. Vol 1, Chap 1, p 30
Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J (2005) Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet 366:129–135
Holzer G, von Skrbensky G, Holzer LA, Pichl W (2009) Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J. Bone Miner. Res. 24:468–474
Verhulp E, van Rietbergen B, Huiskes R (2008) Load distribution in the healthy and osteoporotic human proximal femur during a fall to the side. Bone 42:30–35
Poole KES, Treece GM, Mayhew PM, Vaculik J, Dungl P, Horak M, Šteˇpan JJ (2012) Cortical thickness mapping to identify focal osteoporosis in patients with hip fracture. PLoS ONE 7:e38466
Koga Y (2008) Osteoarthritic of the knee epidemiology, biomechanics, and conservative treatment. Chap 2, pp 41–65 (in Japansese)
Higano Y, Hayami T, Omori G, Koga Y, Endo K, Endo N (2016) The varus alignment and morphologic alterations of proximal tibia affect the onset of medial knee osteoarthritis in rural Japanese women: case control study from the longitudinal evaluation of Matsudai knee osteoarthritis survey. J Orthop Sci 21:166–171
Mochizuki T, Tanifuji O, Koga Y, Sato T, Kobayashi K, Nishino K, Watanabe S, Ariumi A, Fujii T, Yamagiwa H, Omori G, Endo N (2017) Sex differences in femoral deformity determined using three-dimensional assessment for osteoarthritic knees. Knee Surg Sports Traumatol Arthrosc 25:468–476
Mochizuki T, Tanifuji O, Koga Y, Hata R, Mori T, Nishino K, Sato T, Kobayashi K, Omori G, Sakamoto M, Tanabe Y, Endo N (2017) External torsion in a proximal tibia and internal torsion in a distal tibia occur independently in varus osteoarthritic knees compared to healthy knees. J Orthop Sci 22:501–505
Mochizuki T, Tanifuji O, Koga Y, Sato T, Kobayashi K, Watanabe S, Fujii T, Yamagiwa H, Katsumi R, Koga H, Omori G, Endo N (2018) Correlation between posterior tibial slope and sagittal alignment under weight-bearing conditions in osteoarthritic knees. PLoS ONE 13:e0202488
Mochizuki T, Koga Y, Tanifuji O, Sato T, Watanabe S, Koga H, Kobayashi K, Omori G, Endo N (2019) Effect on inclined medial proximal tibial articulation for varus alignment in advanced knee osteoarthritis. J Exp Orthop 6:14. https://doi.org/10.1186/s40634-019-0180-x
Prevrhal S, Engelke K, Kalander WA (1999) Accuracy limits for the determination of cortical width and density: the influence of object size and CT imaging parameters. Phys Med Biol 44:751–764
Prevrhal S, Fox JC, Shepherd JA, Genant HK (2003) Accuracy of CT-based thickness measurement of thin structures: modeling of limited spatial resolution in all three dimensions. Med Phys 30:1–8
Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK (2007) Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 41:505–515
Hangartner TN (2007) Thresholding technique for accurate analysis of density and geometry in QCT, PQCT and CT images. J Musculoskelet Neuronal Interact 7:9–16
Treece GM, Gee AH, Mayhew PM, Poole KE (2010) High resolution cortical bone thickness measurement from clinical CT data. Med Image Anal 14:276–290
Treece GM, Gee AH (2015) Independent measurement of femoral cortical thickness and cortical bone density using clinical CT. Med Image Anal 20:249–264
Gervaise A, Teixeira P, Villani N, Lecocq S, Louis M, Blum A (2012) Dose optimization and reduction if musculoskeletal CT including the spine. In: Tack D, Kalra MK, Gevenois PA (eds) Radiation dose from multidetector CT, 2nd edn. Springer, Heidelberg, pp 369–388
Högler W, Blimkie CJ, Cowell CT, Kemp AF, Briody J, Wiebe P, Farpour-Lambert N, Duncan CS, Woodhead HJ (2003) A comparison of bone geometry and cortical density at the mid-femur between prepuberty and young adulthood using magnetic resonance imaging. Bone 33:771–778
Duda GN, Brand D, Freitag S, Lierse W, Schneider E (1996) Variability of femoral muscle attachments. J Biomech 29:1185–1190
Matsumoto T, Hashimura M, Takayama K, Ishida K, Kawakami Y, Matsuzaki T, Nakano N, Matsushita T, Kuroda R, Kurosaka M (2015) A radiographic analysis of alignment of the lower extremities-initiation and progression of varus-type knee osteoarthritis. Osteoarthritis Cartilage 23:217–223
Katsumi R, Mochizuki T, Sato T, Kobayashi K, Watanabe S, Tanifuji O, Endo N (2018) Contribution of sex and body constitution to three-dimensional lower extremity alignment for healthy, elderly, non-obese humans in a Japanese population. J Exp Orthop 5:32
The third National Health and Nutrition Examination Survey 1988–94. Center of Disease Control website
Alswat KA (2017) Gender disparities in osteoporosis. J Clin Med Res 9:382–387
Bohm ER, Tufescu TV, Marsh JP (2012) The operative management of osteoporotic fractures of the knee: to fix or replace? J Bone Joint Surg [Br] 94:1160–1169
Chang G, Rajapakse CS, Babb JS, Honig SP, Recht MP, Regatte RR (2012) In vivo estimation of bone stiffness at the distal femur and proximal tibia using ultra-high-field 7-Tesla magnetic resonance imaging and micro-finite element analysis. J Bone Miner Metab 30:243–251
Rizzoli R (2010) Microarchitecture in focus. Osteoporos Int 21:S403–S406
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors did not receive and will not receive any benefits or funding from any commercial party related directly or indirectly to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Treece et al. [16] assume that the cortical layer is locally flat and of uniform thickness, at least within the extent of the imaging system’s point spread function (PSF). They can then model the CT values along the line as a convolution of the ‘real’ density with an in-plane and an out-of plane PSF. The real density variation y over distance x is assumed to be piecewise constant, with different values in the surrounding tissue, the cortex and the trabecular bone. This can be expressed as the summation of two step functions:
where y0; y1 and y2 are the CT values in the surrounding tissue, cortical and trabecular bone, respectively, x0 and x1 are the locations of the outer and inner cortical surfaces, and H(x) is a unit step function. The in-plane PSF gi was modeled as a normalized Gaussian function:
where σ represents the extent of the blur. The out-of-plane PSF go is the result of the interaction between the CT slice thickness and the orientation of the cortical layer with respect to the imaging plane. This can be expressed as a rectangular function:
where the extent of blur 2r is calculated from s, the CT slice thickness, and a, the angle the cortical surface normal makes with the imaging plane:
Hence, the fully blurred CT values yblur are given
except for the special case r = 0, when:
The true cortical thickness tr is then:
where tm is the measured in-plane thickness.
About this article
Cite this article
Someya, K., Mochizuki, T., Hokari, S. et al. Age- and sex-related characteristics in cortical thickness of femoral diaphysis for young and elderly subjects. J Bone Miner Metab 38, 533–543 (2020). https://doi.org/10.1007/s00774-019-01079-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01079-9