Abstract
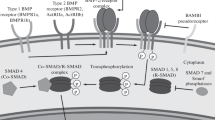
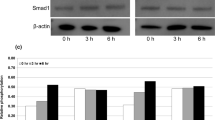
One problem associated with clinical application of CHO-derived recombinant human bone morphogenetic protein (C-BMP-2) is its high cost due to the need for use of high doses. To solve this problem, Escherichia coli-derived BMP-2 (E-BMP-2) has been examined using the technique of molecular unfolding and refolding. However, it is unclear whether the characteristics of E-BMP-2 are appropriate for clinical application. In this study, we examined the biological activity of E-BMP-2 and its heat tolerance in in vitro and in vivo systems. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) confirmed the high purity of E-BMP-2. E-BMP-2-induced alkaline phosphatase expression in osteoprogenitor cells (C2C12, ST2, and primary murine calvarial osteoblast cells) was dose-dependent, and consistently elicited ectopic new ossicles of significant size in mice, also in dose-dependent fashion. In addition, E-BMP-2 induced phosphorylation of Smad1/5/8 and mRNA expression of osteoblastic differentiation markers to the same extent as C-BMP-2. On the other hand, when E-BMP-2 was exposed to increasing heat over time, its bone-inducing capacity was maintained until reaching 70°C for 2 h or 90°C for 15 min. Thus, E-BMP-2 will exhibit a decrease in activity with the sterilization procedures required prior to use in surgery. These findings indicate that the biological capacity and heat stability of E-BMP-2 are almost equivalent to those of currently available C-BMP-2, and suggest that E-BMP-2 might, thus, solve current problems of cost impeding routine clinical use of rhBMP-2.







Similar content being viewed by others
References
Urist MR (1965) Bone: formation by autoinduction. Science 150:893–899
Urist MR, Strates BS (1971) Bone morphogenetic protein. J Dent Res 50:1392–1406
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA (1988) Novel regulators of bone formation: molecular clones and activities. Science 242:1528–1534
Kurihara T, Kitamura K, Takaoka K, Nakazato H (1993) Murine bone morphogenetic protein-4 gene: existence of multiple promoters and exons for the 5′-untranslated region. Biochem Biophys Res Commun 192:1049–1056
Ozkaynak E, Rueger DC, Drier EA, Corbett C, Ridge RJ, Sampath TK, Oppermann H (1990) OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. Embo J 9:2085–2093
Kingsley DM (1994) The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8:133–146
Wozney JM, Rosen V (1998) Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res 346:26–37
Kirker-Head CA (2000) Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev 43:65–92
Namikawa T, Terai H, Suzuki E, Hoshino M, Toyoda H, Nakamura H, Miyamoto S, Takahashi N, Ninomiya T, Takaoka K (2005) Experimental spinal fusion with recombinant human bone morphogenetic protein-2 delivered by a synthetic polymer and beta-tricalcium phosphate in a rabbit model. Spine 30:1717–1722
Wang EA, Rosen V, D’Alessandro JS, Bauduy M, Cordes P, Harada T, Israel DI, Hewick RM, Kerns KM, LaPan P et al (1990) Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA 87:2220–2224
Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM (1996) Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 13:291–300
Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84-A:2123–2134
Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am 83-A(Suppl 1):S151–S158
Burkus JK, Heim SE, Gornet MF, Zdeblick TA (2003) Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech 16:113–122
Ruppert R, Hoffmann E, Sebald W (1996) Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem 237:295–302
Kubler NR, Reuther JF, Faller G, Kirchner T, Ruppert R, Sebald W (1998) Inductive properties of recombinant human BMP-2 produced in a bacterial expression system. Int J Oral Maxillofac Surg 27:305–309
Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T (2000) Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg 38:645–649
Weigel U, Meyer M, Sebald W (1989) Mutant proteins of human interleukin 2. Renaturation yield, proliferative activity and receptor binding. Eur J Biochem 180:295–300
Nakagawa K, Imai Y, Ohta Y, Takaoka K (2007) Prostaglandin E2 EP4 agonist (ONO-4819) accelerates BMP-induced osteoblastic differentiation. Bone 41:543–548
Sugama R, Koike T, Imai Y, Nomura-Furuwatari C, Takaoka K (2006) Bone morphogenetic protein activities are enhanced by 3′, 5′-cyclic adenosine monophosphate through suppression of Smad6 expression in osteoprogenitor cells. Bone 38:206–214
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Saito N, Okada T, Horiuchi H, Murakami N, Takahashi J, Nawata M, Ota H, Nozaki K, Takaoka K (2001) A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol 19:332–335
Kato M, Toyoda H, Namikawa T, Hoshino M, Terai H, Miyamoto S, Takaoka K (2006) Optimized use of a biodegradable polymer as a carrier material for the local delivery of recombinant human bone morphogenetic protein-2 (rhBMP-2). Biomaterials 27:2035–2041
Suzuki A, Terai H, Toyoda H, Namikawa T, Yokota Y, Tsunoda T, Takaoka K (2006) A biodegradable delivery system for antibiotics and recombinant human bone morphogenetic protein-2: a potential treatment for infected bone defects. J Orthop Res 24:327–332
Ohta H, Wakitani S, Tensho K, Horiuchi H, Wakabayashi S, Saito N, Nakamura Y, Nozaki K, Imai Y, Takaoka K (2005) The effects of heat on the biological activity of recombinant human bone morphogenetic protein-2. J Bone Miner Metab 23:420–425
Acknowledgments
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Project Grants 16109009 and 1679085 for KT, and 19791018 for YI).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yano, K., Hoshino, M., Ohta, Y. et al. Osteoinductive capacity and heat stability of recombinant human bone morphogenetic protein-2 produced by Escherichia coli and dimerized by biochemical processing. J Bone Miner Metab 27, 355–363 (2009). https://doi.org/10.1007/s00774-009-0040-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0040-3