Abstract
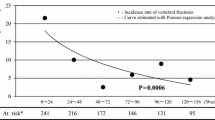
In this multicenter, randomized, double-blind controlled trial, the efficacy and safety of once-weekly dosing with 17.5 mg risedronate was compared with once-daily dosing with 2.5 mg risedronate in Japanese patients with involutional osteoporosis. A total of 496 patients were randomized to receive either once-weekly (n = 249) or once-daily (n = 247) treatment. All patients were supplemented with 200 mg/day calcium. Following 48 weeks of treatment, the mean (±SD) percent changes, from baseline, in the bone mineral density of the lumbar spine (L2-L4 BMD) in the once-weekly and once-daily treatment groups were 5.36 ± 4.27% and 5.87 ± 4.47%, respectively. The difference between the groups was −0.5% (95% confidence interval: −1.35% to 0.35%), demonstrating that the effect on BMD of once-weekly treatment was not inferior to that of once-daily treatment. The time-course reductions in biochemical markers of bone resorption (urinary N- and C-telopeptide of type I collagen) and bone formation (bone-specific alkaline phosphatase) were similar for the two dosing regimens. There were no differences in the incidence of new vertebral fractures or the worsening of existing fractures between the once-weekly (2.2%) and once-daily (2.7%) dosing regimens. No significant differences were observed between the two dosing regimens in the incidence or the type of adverse events. However, 10.1% of the patients in the once-daily group withdrew due to adverse events as compared to 5.2% in the once-weekly group. Moreover, drug-related adverse events, including upper gastrointestinal disorders and abnormal changes in laboratory parameters, tended to be less in the once-weekly dosing regimen than in the once-daily dosing regimen. In conclusion, once-weekly oral dosing with 17.5 mg risedronate was well tolerated in Japanese osteoporotic patients, and showed equivalent efficacy to once-daily oral dosing with 2.5 mg risedronate. This once-weekly regimen is expected to provide a more convenient therapeutic option as an alternative to daily dosing and to enhance patient compliance in long-term therapy for osteoporosis.
Similar content being viewed by others
References
ST Harris NB Watts HK Genant CD McKeever T Hangartner M Keller CH Chesnut SuffixIII J Brown E Eriksen MS Hoseyni DW Axelrod PD Miller (1999) ArticleTitleEffects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial JAMA 282 1344–1352 Occurrence Handle10527181 Occurrence Handle1:CAS:528:DyaK1MXmvFansr0%3D Occurrence Handle10.1001/jama.282.14.1344
J-Y Reginster OH Sorensen M Hooper C Roux ML Brandi B Lund D Ethgen S Pack I Roumagnac R Eastell (2000) ArticleTitleRandomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis Osteoporos Int 11 83–89 Occurrence Handle10663363 Occurrence Handle1:CAS:528:DC%2BD3cXhvFGgsr0%3D Occurrence Handle10.1007/s001980050010
MR McClung P Geusens PD Miller H Zippel WG Bensen C Roux S Adami I Fogelman T Diamond R Eastell PJ Meunier J-Y Reginster (2001) ArticleTitleEffect of risedronate on the risk of hip fracture in elderly women N Engl J Med 344 333–340 Occurrence Handle11172164 Occurrence Handle1:CAS:528:DC%2BD3MXht1Wlu7g%3D Occurrence Handle10.1056/NEJM200102013440503
I Fogelman C Ribot R Smith D Ethgen E Sod J-Y Reginster (2000) ArticleTitleRisedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial J Clin Endocrinol Metab 85 1895–1900 Occurrence Handle10843171 Occurrence Handle1:CAS:528:DC%2BD3cXlt1Wlsb4%3D Occurrence Handle10.1210/jc.85.5.1895
OH Sorensen GM Crawford H Mulder DJ Hosking C Gennari D Mellstrom S Pack D Wenderoth C Cooper J-Y Reginster (2003) ArticleTitleLong-term efficacy of risedronate: a 5-year placebo-controlled clinical experience Bone 32 120–126 Occurrence Handle12633783 Occurrence Handle1:CAS:528:DC%2BD3sXhvFWqu7o%3D Occurrence Handle10.1016/S8756-3282(02)00946-8
DD Mellstrom OH Sorensen S Goemaere C Roux TD Johmson AA Chines (2004) ArticleTitleSeven years of treatment with risedronate in women with postmenopausal osteoporosis Calcif Tissue Int 75 462–468 Occurrence Handle15455188 Occurrence Handle1:CAS:528:DC%2BD2MXksVGqug%3D%3D Occurrence Handle10.1007/s00223-004-0286-7
M Fukunaga K Kushida H Kishimoto M Shiraki Y Taketani H Minaguchi T Inoue R Morita H Morii K Yamamoto Y Ohashi H Orimo (2002) ArticleTitleA comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial Osteoporos Int 13 971–979 Occurrence Handle12459940 Occurrence Handle1:CAS:528:DC%2BD38XptFGitrY%3D Occurrence Handle10.1007/s001980200135
K Kushida M Fukunaga H Kishimoto M Shiraki A Itabashi T Inoue K Kaneda H Morii H Nawata K Yamamoto Y Ohashi H Orimo (2004) ArticleTitleA comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial J Bone Miner Metab 22 470–479
Y Ogura A Gonsho Orimo H Cyong J-C (2004) ArticleTitleClinical trial of risedronate in Japanese volunteers: single and multiple oral dose studies J Bone Miner Metab 22 111–119 Occurrence Handle14999521 Occurrence Handle1:CAS:528:DC%2BD2cXhsl2ht7s%3D Occurrence Handle10.1007/s00774-003-0458-y
DY Mitchell RA Eusebio NA Sacco-Gibson KA Pallone SC Kelly JD Nesbitt CP Brezovic GA Thompson JH Powell (2000) ArticleTitleDose-proportional pharmacokinetics of risedronate on single-dose oral administration to healthy volunteers J Clin Pharmacol 40 258–265 Occurrence Handle10709154 Occurrence Handle1:CAS:528:DC%2BD3cXivF2ksL0%3D Occurrence Handle10.1177/00912700022008928
M Shiraki M Fukunaga K Kushida H Kishimoto Y Taketani H Minaguchi T Inoue R Morita H Morii K Yamamoto Y Ohashi H Orimo (2003) ArticleTitleA double-blind dose-ranging study of risedronate in Japanese patients with osteoporosis (a study by the Risedronate Late Phase II Research Group) Osteoporos Int 14 225–234 Occurrence Handle12730746 Occurrence Handle1:CAS:528:DC%2BD3sXlslOls7c%3D
JH Lin (1996) ArticleTitleBisphosphonates: a review of their pharmacokinetic properties Bone 18 75–85 Occurrence Handle8833200 Occurrence Handle1:CAS:528:DyaK28XisVGjs7g%3D Occurrence Handle10.1016/8756-3282(95)00445-9
JG Seedor HA Quartuccio DD Thompson (1991) ArticleTitleThe bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats J Bone Miner Res 6 339–346 Occurrence Handle1858520 Occurrence Handle1:CAS:528:DyaK3MXks1Cnu7c%3D Occurrence Handle10.1002/jbmr.5650060405
F Bauss M Wagner LH Hothorn (2002) ArticleTitleTotal administered dose of ibandronate determines its effects on bone mass and architecture in ovariectomized aged rats J Rheumatol 29 990–998 Occurrence Handle12022363 Occurrence Handle1:CAS:528:DC%2BD38XktlShs7g%3D
JP Brown MR Kendler MR McClung RD Emkey JD Adachi MA Bolognese Z Li A Balske R Lindsay (2002) ArticleTitleThe efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis Calcif Tissue Int 71 103–111 Occurrence Handle12085156 Occurrence Handle1:CAS:528:DC%2BD38Xms1emurY%3D Occurrence Handle10.1007/s00223-002-2011-8
NB Watts R Lindsay Z Li C Kasibhatla J Brown (2003) ArticleTitleUse of matched historical controls to evaluate the anti-fracture efficacy of once-a-week risedronate Osteoporos Int 14 437–441 Occurrence Handle12730756 Occurrence Handle1:CAS:528:DC%2BD3sXlsVOnsb8%3D Occurrence Handle10.1007/s00198-003-1401-8
T Schnitzer HG Bone G Crepaldi S Adami M McClung et al. (2000) ArticleTitleTherapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis Aging Clin Exp Res 12 1–12 Occurrence Handle1:CAS:528:DC%2BD3cXitVCns7g%3D
R Rizzoli SL Greenspan HG Bone SuffixIII TJ Schnitzer NB Watts S Adami AJ Foldes C Roux MA Levine B Uebelhart AC Santora SuffixII A Kaur CA Peverly JJ Orloff (2002) ArticleTitleTwo-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopaual osteoporosis J Bone Miner Res 17 1988–1996 Occurrence Handle12412806 Occurrence Handle1:STN:280:DC%2BD38nltFGnug%3D%3D Occurrence Handle10.1359/jbmr.2002.17.11.1988
Y Ouchi H Orimo (1989) ArticleTitleThe disease of the old age and metabolic change of calcium and magnesium (in Japanese) Jpn J Geriat 26 216–221 Occurrence Handle1:STN:280:DyaK3c%2Fht1OgsQ%3D%3D
H Orimo Y Sugioka M Fukunaga Y Muto T Hotokebuchi I Gorai T Nakamura K Kushida H Tanaka T Ikai Y Oh-hashi (1998) ArticleTitleDiagnostic criteria of primary osteoporosis J Bone Miner Metab 16 139–150 Occurrence Handle10.1007/s007740050038
H Orimo Y Hayashi M Fukunaga T Sone S Fujiwara M Shiraki K Kushida S Miyamoto S Soen J Nishimura Y Oh-hashi T Hosoi I Gorai H Tanaka T Igai H Kishimoto (2001) ArticleTitleDiagnostic criteria for primary osteoporosis: year 2000 revision J Bone Miner Metab 19 331–337 Occurrence Handle11685647 Occurrence Handle1:STN:280:DC%2BD3MngvFGntw%3D%3D Occurrence Handle10.1007/s007740170001
Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis. Rockville, Division of Metabolic and Endocrine Drug Products Food and Drug Administration (1994)
D Uebelhart E Gineyts M-C Chapuy PD Delmas (1990) ArticleTitleUrinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone disease Bone Miner 8 87–96 Occurrence Handle2106358 Occurrence Handle1:CAS:528:DyaK3cXlsFSjtr8%3D Occurrence Handle10.1016/0169-6009(91)90143-N
R Eastell I Barton RA Hannon A Chimes P Garnero PD Delmas (2003) ArticleTitleRelationship of early changes in bone resorption to the reduction in fracture risk with risedronate J Bone Miner Res 18 1051–1056 Occurrence Handle12817758 Occurrence Handle1:CAS:528:DC%2BD3sXkslOhsb0%3D Occurrence Handle10.1359/jbmr.2003.18.6.1051
SL Greenspan HN Rosen RA Parker (2000) ArticleTitleEarly changes in serum N-telopeptide and C-telopeptide cross-linked collagen type 1 predict long-term response to alendronate therapy in elderly women J Clin Endocrinol Metab 85 3537–3540 Occurrence Handle11061497 Occurrence Handle1:CAS:528:DC%2BD3cXnsFOgsbY%3D Occurrence Handle10.1210/jc.85.10.3537
P Ganero WJ Shih E Gineyts DB Karpf PD Delmas (1994) ArticleTitleComparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment J Clin Endocrinol Metab 79 1693–1700 Occurrence Handle10.1210/jc.79.6.1693
HG Bone S Adami R Rizzoli M Favus PD Ross A Santra S Praharada A Daifotis J Orloff J Yates (2000) ArticleTitleWeekly administration of alendronate: rationale and plan for clinical assessment Clin Ther 22 15–28 Occurrence Handle10688387 Occurrence Handle1:CAS:528:DC%2BD3cXhsVaqt7s%3D Occurrence Handle10.1016/S0149-2918(00)87974-6
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kishimoto, H., Fukunaga, M., Kushida, K. et al. Efficacy and tolerability of once-weekly administration of 17.5 mg risedronate in Japanese patients with involutional osteoporosis: a comparison with 2.5-mg once-daily dosage regimen. J Bone Miner Metab 24, 405–413 (2006). https://doi.org/10.1007/s00774-006-0706-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00774-006-0706-z