Abstract
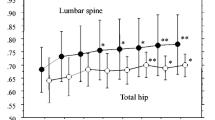
The efficacy and safety of treatment with oral alendronate (ALN) 35 mg once weekly for 52 weeks were compared with those of ALN 5 mg once daily in a double-blind, randomized, multicenter study of Japanese patients with involutional osteoporosis. The primary efficacy end point was the percent change from baseline in the lumbar spine (L1–L4) bone mineral density (BMD) after 52 weeks of treatment. In this study, 328 patients were randomized to ALN 5 mg once daily (160 patients) or ALN 35 mg once weekly (168 patients). The adjusted mean percent change from baseline in lumbar spine (L1–L4) BMD after 52 weeks of treatment was 5.8% and 6.4% in the once-daily group and the once-weekly group, respectively (both P < 0.001). The 95% confidence interval for the difference in spine BMD change between the two treatment groups was −0.31% to 1.48%, indicating that the two regimens were therapeutically equivalent, since the confidence interval fell entirely within the predefined equivalence criterion (±1.5%). The time course of the spine BMD increase was also similar for both regimens. Regarding total hip BMD, mean changes from baseline at 52 weeks were 2.8% and 3.0% in the once-daily group and the once-weekly group, respectively. In addition, the bone markers (urinary deoxypyridinoline, urinary type-I collagen N-telopeptides, and serum bone-specific alkaline phosphatase) were reduced to a similar level by either treatment throughout the treatment period. The tolerability and safety profiles were also similar between the treatment groups. Taken together, we conclude that the efficacy and safety of the ALN 35-mg once-weekly regimen are therapeutically equivalent to those of the ALN 5-mg once-daily regimen.
Similar content being viewed by others
References
InstitutionalAuthorNameConsensus Development Conference (1993) ArticleTitleDiagnosis, prophylaxis, and treatment of osteoporosis Am J Med 94 646–650
InstitutionalAuthorNameNIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) ArticleTitleOsteoporosis prevention, diagnosis, and therapy JAMA 285 785–795
InstitutionalAuthorNameJapan Osteoporosis Society Working Group (2002) ArticleTitleGuideline to Clinical Evaluation Methods for Agents Used in Treatment of Osteoporosis, 2002 Revised Edition (in Japanese) Osteoporos Jpn 10 7–79
S Fujiwara F Kasagi N Masunari K Naito G Suzuki M Fukunaga (2003) ArticleTitleFracture prediction from bone mineral density in Japanese men and women J Bone Miner Res 18 1547–1553 Occurrence Handle12929946
DM Black SR Cummings DB Karpf JA Cauley DE Thompson MC Nevitt DC Bauer HK Genant WL Haskell R Marcus SM Ott JC Torner SA Quandt TF Reiss KE Ensrud (1996) ArticleTitleRandomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures Lancet 348 1535–1541 Occurrence Handle8950879
SR Cummings DM Black DE Thompson WB Applegate E Barrett-Connor TA Musliner L Palermo R Prineas SM Rubin JC Scott T Vogt R Wallace AJ Yates AZ LaCroix (1998) ArticleTitleEffect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the fracture intervention trial JAMA 280 2077–2082 Occurrence Handle10.1001/jama.280.24.2077 Occurrence Handle9875874
UA Liberman SR Weiss J Broll HW Minne H Quan NH Bell J Rodriguez-Portales RW Downs SuffixJr J Dequeker M Favus E Seeman RR Recker T Capizzi ACII Santora A Lombardi RV Shah LJ Hirsch DB Karpf (1995) ArticleTitleEffect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis N Engl J Med 333 1437–1443 Occurrence Handle10.1056/NEJM199511303332201
JR Tucci RP Tonino RD Emkey CA Peverly U Kher AC Santora SuffixII (1996) ArticleTitleEffect of three years of oral alendronate treatment in postmenopausal women with osteoporosis Am J Med 101 488–501 Occurrence Handle10.1016/S0002-9343(96)00282-3 Occurrence Handle8948272
JP Devogelaer H Broll R Correa-Rotter DC Cumming C Nagant de Deuxchaisnes et al. (1996) ArticleTitleOral alendronate induces progressive increases in bone mass of the spine, hip, and total body over 3 years in postmenopausal women with osteoporosis Bone 18 141–150 Occurrence Handle10.1016/8756-3282(95)00436-X Occurrence Handle8833208
DB Karpf DR Shapiro E Seeman KE Ensrud CC Johnston SuffixJr S Adami ST Harris ACII Santora LJ Hirsch L Oppenheimer D Thompson (1997) ArticleTitlePrevention of nonvertebral fractures by alendronate. A meta-analysis JAMA 277 1159–1164 Occurrence Handle10.1001/jama.277.14.1159 Occurrence Handle9087473
M Sharpe S Noble CM Spencer (2001) ArticleTitleAlendronate: an update of its use in osteoporosis Drugs 61 999–1039 Occurrence Handle11434454
A Cranney G Wells A Willan L Griffith N Zytaruk V Robinson D Black J Adachi B Shea P Tugwell G Guyatt (2002) ArticleTitleII. Meta-analysis of alendronate for the treatment of postmenopausal women Endocr Rev 23 508–516 Occurrence Handle10.1210/er.2001-2002 Occurrence Handle12202465
RP Tonino PJ Meunier R Emkey JA Rodriguez-Portales CJ Menkes RD Wasnich HG Bone AC Santora M Wu R Desai PD Ross (2000) ArticleTitleSkeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group J Clin Endocrinol Metab 85 3109–3115 Occurrence Handle10.1210/jc.85.9.3109 Occurrence Handle10999794
HA Pols D Felsenberg DA Hanley J Stepán M Muñoz-Torres TJ Wilkin G Qin-sheng AM Galich K Vandormael AJ Yates B Stych (1999) ArticleTitleMultinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study Osteoporos Int 9 461–468 Occurrence Handle10550467
DM Black DE Thompson DC Bauer K Ensrud T Musliner MC Hochberg MC Nevitt S Suryawanshi SR Cummings (2000) ArticleTitleFracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial J Clin Endocrinol Metab 85 4118–4124 Occurrence Handle10.1210/jc.85.11.4118 Occurrence Handle11095442
HG Bone D Hosking JP Devogelaer JR Tucci RD Emkey RP Tonino JA Rodriguez-Portales RW Downs J Gupta AC Santora UA Liberman (2004) ArticleTitleTen years’ experience with alendronate for osteoporosis in postmenopausal women N Engl J Med 350 1189–1199 Occurrence Handle10.1056/NEJMoa030897 Occurrence Handle15028823
LE Wehren D Hosking MC Hochberg (2004) ArticleTitlePutting evidence-based medicine into clinical practice: comparing anti-resorptive agents for the treatment of osteoporosis Curr Med Res Opin 20 525–531 Occurrence Handle10.1185/030079904125003269 Occurrence Handle15119990
M Shiraki K Kushida M Fukunaga H Kishimoto K Kaneda H Minaguchi T Inoue A Tomita Y Nagata M Nakashima H Orimo (1998) ArticleTitleA placebo-controlled, single-blind study to determine the appropriate alendronate dosage in postmenopausal Japanese patients with osteoporosis Endocr J 45 191–201 Occurrence Handle9700472
T Nakamura K Kushida M Shiraki M Fukunaga M Taga H Kishimoto A Tomita T Inoue H Minaguchi K Kaneda Y Nagata M Nakashima H Orimo (1998) ArticleTitleA double-blind, dose-ranging study of alendronate in patients with involutional osteoporosis, osteoporotic osteopenia or artificial menopause (in Japanese) Med Consult New Remedy 35 3–17
H Kishimoto M Shiraki M Fukunaga K Kushida K Kaneda T Inoue A Tomita K Yamamoto H Orimo (1998) ArticleTitleA long-term study of alendronate in patients with involutional osteoporosis (in Japanese) Med Consult New Remedy 35 19–41
M Shiraki K Kushida M Fukunaga H Kishimoto M Taga T Nakamura K Kaneda H Minaguchi T Inoue H Morii A Tomita K Yamamoto Y Nagata M Nakashima H Orimo (1999) ArticleTitleA double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis Osteoporos Int 10 183–192 Occurrence Handle10.1007/s001980050214 Occurrence Handle10525709
K Kushida M Shiraki T Nakamura H Kishimoto H Morii K Yamamoto K Kaneda M Fukunaga T Inoue M Nakashima H Orimo (2002) ArticleTitleThe efficacy of alendronate in reducing the risk for vertebral fracture in Japanese patients with osteoporosis: a randomized, double-blind, active-controlled, double-dummy trial Curr Ther Res 63 606–620 Occurrence Handle10.1016/S0011-393X(02)80065-0
K Kushida M Shiraki T Nakamura H Kishimoto H Morii K Yamamoto K Kaneda M Fukunaga T Inoue M Nakashima H Orimo (2004) ArticleTitleAlendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study J Bone Miner Metab 22 462–468 Occurrence Handle10.1007/s00774-004-0508-0 Occurrence Handle15316867
T Schnitzer HG Bone G Crepaldi S Adami M McClung et al. (2000) ArticleTitleTherapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis Aging Clin Exp Res 12 1–12
R Rizzoli SL Greenspan H Bone SuffixIII TJ Schnitzer NB Watts S Adami AJ Foldes C Roux MA Levine B Uebelhart AC Santora SuffixII A Kaur CA Peverly JJ Orloff (2002) ArticleTitleTwo-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis J Bone Miner Res 17 1988–1996 Occurrence Handle12412806
S Naruse Y Kato K Tani A Hisaka H Watanabe H Arizono T Taniguchi (2004) ArticleTitlePharmacokinetic study of alendronate in postmenopausal Japanese women – Evaluation of urinary excretion and safety profile of alendronate after single oral administration of 35 mg and 5 mg tablet formulations (in Japanese) Rinsyo Iyaku 20 1–8
RN Greenberg (1984) ArticleTitleOverview of patient compliance with medication dosing: a literature review Clin Ther 6 592–599 Occurrence Handle6383611
HG Bone S Adami R Rizzoli M Favus PD Ross A Santora S Prahalada A Daifotis J Orloff J Yates (2000) ArticleTitleWeekly administration of alendronate: rationale and plan for clinical assessment Clin Ther 22 15–28 Occurrence Handle10.1016/S0149-2918(00)87974-6 Occurrence Handle10688387
F Lanza B Sahba H Schwartz S Winograd J Torosis H Quan R Reyes T Musliner A Daifotis A Leung (2002) ArticleTitleThe upper GI safety and tolerability of oral alendronate at a dose of 70 milligrams once weekly: a placebo-controlled endoscopy study Am J Gastroenterol 97 58–64 Occurrence Handle10.1111/j.1572-0241.2002.05446.x Occurrence Handle11808969
S Greenspan E Field-Munves R Tonino M Smith R Petruschke L Wang J Yates AE DePapp J Palmisano (2002) ArticleTitleTolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study Mayo Clin Proc 77 1044–1052 Occurrence Handle12374248
B Cryer DC Bauer (2002) ArticleTitleOral bisphosphonates and upper gastrointestinal tract problems: what is the evidence? Mayo Clin Proc 77 1031–1043 Occurrence Handle12374247
JA Simon EM Lewiecki ME Smith RA Petruschke L Wang JJ Palmisano (2002) ArticleTitlePatient preference for once-weekly alendronate 70 mg versus once-daily alendronate 10 mg: a multicenter, randomized, open-label, crossover study Clin Ther 24 1871–1886 Occurrence Handle10.1016/S0149-2918(02)80085-6 Occurrence Handle12501880
D Kendler AWC Kung GEH Fuleihan JGG Gonzalez KA Gaines N Verbruggen ME Melton (2004) ArticleTitlePatients with osteoporosis prefer once weekly to once daily dosing with alendronate Eur Menopause J 48 243–251
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Uchida, S., Taniguchi, T., Shimizu, T. et al. Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab 23, 382–388 (2005). https://doi.org/10.1007/s00774-005-0616-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00774-005-0616-5