Summary.
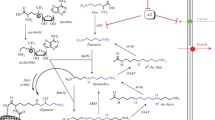
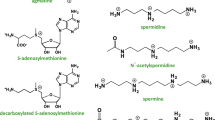
Owing to the establishment of cells and transgenic animals which either lack or over-express acetylCoA:spermidine N1-acetyltransferase a major progress was made in our understanding of the role of polyamine acetylation. Cloning of polyamine oxidases of mammalian cell origin revealed the existence of several enzymes with different substrate and molecular properties. One appears to be identical with the polyamine oxidase that was postulated to catalyse the conversion of spermidine to putrescine within the interconversion cycle. The other oxidases are presumably spermine oxidases, because they prefer free spermine to its acetyl derivatives as substrate. Transgenic mice and cells which lack spermine synthase revealed that spermine is not of vital importance for the mammalian organism, but its transformation into spermidine is a vitally important reaction, since in the absence of active polyamine oxidase, spermine accumulates in blood and causes lethal toxic effects.
Numerous metabolites of putrescine, spermidine and spermine, which are presumably the result of diamine oxidase-catalysed oxidative deaminations, are known as normal constituents of organs of vertebrates and of urine. Reasons for the apparent contradiction that spermine is in vitro a poor substrate of diamine oxidase, but is readily transformed into N8-(2-carboxyethyl)spermidine in vivo, will need clarification.
Several attempts were made to establish diamine oxidase as a regulatory enzyme of polyamine metabolism. However, diamine oxidase has a slow turnover. This, together with the efficacy of the homeostatic regulation of the polyamines via the interconversion reactions and by transport pathways renders a role of diamine oxidase in the regulation of polyamine concentrations unlikely. 4-Aminobutyric acid, the product of putrescine catabolism has been reported to have antiproliferative properties. Since ornithine decarboxylase and diamine oxidase activities are frequently elevated in tumours, it may be hypothesised that diamine oxidase converts excessive putrescine into 4-aminobutyric acid and thus restricts tumour growth and prevents malignant transformation. This function of diamine oxidase is to be considered as part of a general defence function, of which the prevention of histamine and cadaverine accumulation from the gastrointestinal tract is a well-known aspect.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seiler, N. Catabolism of polyamines. Amino Acids 26, 217–233 (2004). https://doi.org/10.1007/s00726-004-0070-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-004-0070-z