Abstract
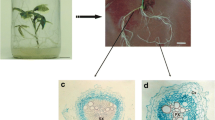
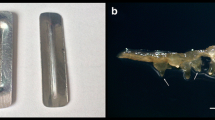
Adventitious root cultures of Tarenaya rosea were successfully cryopreserved using the encapsulation-vitrification technique. Histological analysis revealed useful information on the successive steps of cryopreservation. Coupled with complementary histochemical approaches, these studies provided cellular and tissue descriptions of T. rosea root cultures during cryopreservation and contributed to an understanding of cellular stress responses, as well as characterization of the anatomical pattern of root regeneration. The effects of exposure duration to PVS3 solution (0–120 min), unloading treatment (direct and gradual), and recovery medium (liquid and solid) on recovery of cryopreserved roots were investigated. The highest recovery (91%) after cooling in liquid nitrogen (LN) was reached with PVS3 treatment for 90 min, gradual rehydration in unloading solution, and recovery on solid MS medium. The cryopreserved roots showed high multiplication capacity, which was maintained for up to four subcultures. The effect of cryopreservation on root structure was investigated by histological and histochemical studies. Plasmolysis intensified during exposure to loading and PVS3 solutions, but decreased after unloading treatment. The proportion of intercellular spaces increased progressively throughout the cryopreservation protocol, culminating in root cortex disruption. Histochemical analyses revealed polysaccharides, proteins, and both lipidic and pectic substances in intercellular spaces. The vascular cylinder remained intact, ensuring the formation of new roots from the pericycle, showing that proliferative capacity of cryopreserved roots had not diminished.






Similar content being viewed by others
References
Albarello N, Ribeiro IG, Simões C, Castro TC, Gianfaldoni MG, Callado C, Kuster RM, Coelho MGP, Mansur E (2007) Histological analysis of calluses from in vitro propagated plants of Cleome spinosa Jacq. Rev Bras Biocienc 5:699–701
Alla-N’nan O, Tiécoura K, Bi SG, Verdeil J-L, Malaurie B (2014) Ultrastructural changes during cryopreservation of plumules and embryos of coconut (Cocos nucifera L.). Int J Agro Agric Res 5(6):103–115
Antony JJJ, Mubbarakh SA, Mahmood M, Subramaniam S (2014) Effect of plasmolysis on protocorm-like bodies of Dendrobium Bobby Messina orchid following cryopreservation with encapsulation–dehydration method. Appl Biochem Biotechnol 172:1433–1444
Barraco G, Sylvestre I, Collin M, Escoute J, Lartaud M, Verdeil JL, Engelmann F (2014) Histocytological analysis of yam (Dioscorea alata) shoot tips cryopreserved by encapsulation–dehydration. Protoplasma 251:177–189
Benson EE (1990) Free radical damage in stored plant germplasm. International Board for Plant Genetic Resources, Rome
Benson EE, Bremner D (2004) Oxidative stress in the frozen plant: a free radical point of view. In: Fuller BJ, Lane N, Benson EE (eds) Life in the frozen state. CRC Press LLC, United States of America, pp 205–241
Benson EE, Hamill JD (1991) Cryopreservation and post freezing molecular and biosynthetic stability in transformed roots of Beta vulgaris and Nicotiana rustica. Plant Cell Tissue Organ Cult 24:163–172
Buffard-Morel J, Verdeil JL, Pannetier C (1992) Embryogenèse somatique du cocotier (Cocos nucifera L.) à partir d’explants foliaires: étude histologique. Can J Bot 70:735–741
Cordeiro LS, Simões-Gurgel C, Albarello N (2015) Multiplication and cryopreservation of adventitious roots of Cleome rosea Vahl. In Vitro Cell Dev Biol Plant 51:249–257
Cordeiro LS, Simões-Gurgel C, Albarello N (2016) Cryopreservation of adventitious roots of Cleome rosea Vahl (Cleomaceae) using a vitrification technique and assessment of genetic stability. CryoLetters 37(4):231–242
Cruz-Cruz CA, González-Arnao MT, Engelmann F (2013) Biotechnology and conservation of plant biodiversity. Resources 2:73–95
Ding F, Jin S, Hong N, Zhong Y, Cao Q, Yi G, Wang G (2008) Vitrification–cryopreservation, an efficient method for eliminating Candidatus Liberobacter asiaticus, the citrus Huanglongbing pathogen, from in vitro adult shoot tips. Plant Cell Rep 27:241–250
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol Plant 47:5–16
Engelmann F, Gonzalez-Arnao MT (2013) Introducción a la conservación ex situ de los recursos genéticos vegetales. In: Gonzalez-Arnao MT, Engelmann F (eds) Crioconservación de plantas en América Latina y el Caribe. Instituto Interamericano de Cooperación para la Agricultura (IICA), San José, pp 25–35
Fang JY, Wetten A (2011) Importance of structural integrity of somatic embryos for long-term cryopreservation of cocoa (Theobroma cacao L.) germplasm. Afr J Agric Res 6(17):3954–3961
Fisher DB (1968) Protein staining of ribboned epon sections for light microscopy. Histochem 16:92–96
Fraga HPF, Vieira LN, Puttkammer CC, Silva JM, Anjos KG, Oliveira EM, Guerra MP (2016) High-efficiency cryopreservation of Araucaria angustifolia (Bertol.) Kuntze embryogenic cultures: ultrastructural characterization and morpho-physiological features. Plant Cell Tissue Organ Cult 124:307–318
Gerlach D (1984) Botanische mikrotechnik. Thieme Verlag, Stuttgart
Gogoi K, Kumaria S, Tandon P (2012) A comparative study of vitrification and encapsulation-vitrification for cryopreservation of protocorms of Cymbidium eburneum L., a threatened and vulnerable orchid of India. Cryoletters 33:443–452
Gonzalez-Arnao MT, Panta A, Roca WM, Escobar RH, Engelmann F (2008) Development and large scale application of cryopreservation techniques for shoot and somatic embryo cultures of tropical crops. Plant Cell Tissue Organ Cult 92:1–13
Harding K, Benson EE, Nunes EC, Pilatti FK, Lemos J, Viana AM (2013) Can biospecimen science expedite the ex situ conservation of plants in megadiverse countries? A focus on the flora of Brazil. Crc Cr Rev Plant Sci 32(6):411–444
Hirata K, Mukai M, Goda S, Ishio-Kinugasa M, Yoshida K, Sakai A, Miyamoto K (2002) Cryopreservation of hairy root cultures of Vinca minor (L.) by encapsulation-dehydration. Biotechnol Lett 24:371–376
Jensen WA (1962) Botanical histochemistry: principle and practice. W. H. Freeman, San Francisco
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Jung DW, Sung CK, Touno K, Yoshimatsu K, Shimomura K (2001) Cryopreservation of Hyoscyamus niger adventitious roots by vitrification. J Plant Physiol 158:801–805
Kim HH, Popova EV, Yi JY, Cho GT, Park SU, Lee SC, Engelmann F (2010) Cryopreservation of hairy roots of Rubia akane (Nakai) using a droplet-vitrification procedure. CryoLetters 31:473–484
Le KC, Kim HH, Park SY (2019) Modification of the droplet-vitrification method of cryopreservation to enhance survival rates of adventitious roots of Panax ginseng. Hortic Environ Biotechnol 60:501–510
Martín C, Kremer C, González I, González-Benito ME (2015) Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tissue Organ Cult 122(1):185–195
Mathew L, McLachlan A, Jibran R, Burritt DJ, Pathirana R (2018) Cold, antioxidant and osmotic pre-treatments maintain the structural integrity of meristematic cells and improve plant regeneration in cryopreserved kiwifruit shoot tips. Protoplasma 255(4):1065–1077
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Dandin VS, Paek KY (2016) Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem Rev 15:129–145
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Oh SY, Wu CH, Popova E, Hahn EJ, Paek KY (2009) Cryopreservation of Panax ginseng adventitious roots. J Plant Biol 52:348–354
Park SU, Kong H, Shin DJ, Bae CH, Lee SC, Bae CH, Rha ES, Kim HH (2014) Development of vitrification protocol in Rubia akane (Nakai) hairy roots using a systematic approach. CryoLetters 35(2):138–144
Pasqua G, Monacelli B, Valletta A, Santamaria AR, Fiorillo F (2005) Synthesis and/or accumulation of bioactive molecules in the in vivo and in vitro root. Plant Biosyst 139(2):180–188
Poobathy R, Sinniah UR, Xavier R, Subramaniam S (2013) Catalase and superoxide dismutase activities and the total protein content of protocorm-like bodies of Dendrobium sonia-28 subjected to vitrification. Appl Biochem Biotechnol 170:1066–1079
Popova E, Paek KY, Kim HH (2011) Cryopreservation of medicinal plants: the case of in vitro cultures. In: Kumar A, Roy S (eds) Plant tissue culture and applied plant biotechnology. Aavishkar Publishers, Jaipur, pp 153–196
Rocha AS, Rocha EK, Alves LM, Moraes BA, Castro TC, Albarello N, Simões-Gurgel C (2015) Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J Plant Biochem Biotechnol 24:105–113
Rubinsky B (2003) Principles of low temperature cell preservation. Heart Fail Rev 8:277–284
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28(3):151–172
Sakai A, Hirai D, Niino T (2008) Development of PVS-based vitrification protocols. In: Reed BM (ed) Plant cryopreservation–a practical guide. Springer, New York, pp 33–57
Salma M, Engelmann-Sylvestre I, Collin M, Escoute J, Lartaud M, Yi JY, Kim HH, Verdeil JL, Engelmann F (2014) Effect of the successive steps of a cryopreservation protocol on the structural integrity of Rubia akane Nakai hairy roots. Protoplasma 251(3):649–659
Scarano FR (2009) Plant communities at the periphery of the Atlantic rain forest: rare-species bias and its risks for conservation. Biol Conserv 142:1201–1208
Sershen BP, Pammenter NW, Wesley-Smith J (2012) The effects of various parameters during processing for cryopreservation on the ultrastructure and viability of recalcitrant zygotic embryos of Amaryllis belladonna. Protoplasma 249:155–169
Shin DJ, Lee HE, Bae CH, Park SU, Kang HN, Kim HH (2014) Development of an encapsulation-vitrification protocol for Rubia akane (Nakai) hairy roots: a comparison with non-encapsulation. CryoLetters 35(5):377–384
Simão MJ, Collin M, Garcia RO, Mansur E, Pacheco G, Engelmann F (2018) Histological characterization of Passiflora pohlii Mast. root tips cryopreserved using the V-Cryo-plate technique. Protoplasma 255(3):741–750
Simões C, Santos AS, Albarello N, Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). J Plant Biotechnol 6:199–204
Simões C, Mattos JCP, Sabino KCC, Caldeira-De-Araújo A, Coelho MGP, Albarello N, Figueiredo SFL (2006) Medicinal potential from in vivo and acclimatized plants of Cleome rosea. Fitoterapia 77:94–99
Simões C, Albarello N, Callado CH, Castro TC, Mansur E (2009a) New approaches for shoot production and establishment of in vitro root cultures of Cleome rosea Vahl. Plant Cell Tissue Organ Cult 98:79–86
Simões C, Bizarri CHB, Cordeiro LS, Castro TC, Coutada LM, Silva AJR, Albarello N, Mansur E (2009b) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol Biochem 47:895–903
Simões C, Albarello N, Callado CH, Castro TC, Mansur E (2010a) Somatic embryogenesis and plant regeneration from callus cultures of Cleome rosea Vahl. Braz Arch Biol Technol 53:679–686
Simões C, Castro TC, Cordeiro LS, Albarello N, Mansur E, Romanos MTV (2010b) Antiviral activity of Cleome rosea extracts from field-grown plants and tissue culture-derived materials against acyclovir-resistant Herpes simplex viruses type 1 (ACVr-HSV-1) and type 2 (ACVr-HSV-2). World J Microbiol Biotechnol 26:93–99
Simões-Gurgel C, Cordeiro LS, Castro TC, Callado CH, Albarello N, Mansur E (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tissue Organ Cult 106:537–545
Simões-Gurgel C, Rocha AS, Cordeiro LS, Gayer CRM, Castro TC, Coelho MGP, Albarello N, Mansur E, Rosa ACP (2012) Antibacterial activity of field-grown plants, in vitro propagated plants, callus and cell suspension cultures of Cleome rosea Vahl. J Pharm Res 5:3304–3308
Singh S, Pandey P, Ghosh S, Banerjee S (2018) Anti-cancer labdane diterpenoids from adventitious roots of Andrographis paniculata: augmentation of production prospect endowed with pathway gene expression. Protoplasma 255(5):1387–1400
Skyba M, Urbanová M, Kapchina-Toteva V, Kosuth J, Harding K, Cellárová E (2010) Physiological, biochemical and molecular characteristics of cryopreserved Hypericum perforatum L. shoot tips. CryoLetters 31(3):249–260
Ślesak H, Góralski G, Kwolek D, Dziedzic K, Grabowska-Joachimiak A (2015) Male adventitious roots of Rumex thyrsiflorus Fingerh. as a source of genetically stable micropropagated plantlets. Plant Cell Tissue Organ Cult 123:193–203
Soares Neto RL, Thomas WW, Barbosa MRV, Roalson EH (2018) New combinations and taxonomic notes for Tarenaya (Cleomaceae). Acta Bot Bras 32:540–545
Stewart P, Taylor M, Mycock D (2001) The sequence of the preparative procedures affects the success of cryostorage of cassava somatic embryos. CryoLetters 22:35–42
Teoh KH, Weathers PJ, Cheetham RD, Walcerz DB (1996) Cryopreservation of transformed (hairy) roots of Artemisia annua. Cryobiology 33:106–117
Touno K, Yoshimatsu K, Shimomura K (2006) Characteristics of Atropa belladonna hairy roots cryopreserved by vitrification method. CryoLetters 27:65–72
Volk GM, Caspersen AM (2007) Plasmolysis and recovery of different cell types in cryoprotected shoot tips of Mentha x piperita. Protoplasma 231:215–226
Wen B, Cai C, Wang R, Song S, Song J (2012) Cytological and physiological changes in recalcitrant Chinese fan palm (Livistona chinensis) embryos during cryopreservation. Protoplasma 249(2):323–335
Wolfe J, Bryant G (2001) Cellular cryobiology: thermodynamic and mechanical effects. Int J Refrig 24:438–450
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tissue Organ Cult 92:251–260
Yang X, Popova E, Shukla MR, Saxena PK (2019) Root cryopreservation to biobank medicinal plants: a case study for Hypericum perforatum L. In Vitro Cell Dev Biol Plant 55(4):392–402
Yi JY, Sylvestre I, Collin M, Salma M, Lee SY, Kim HH, Park HJ, Engelmann F (2012) Improved cryopreservation using droplet-vitrification and histological changes associated with cryopreservation of madder (Rubia akane Nakai). Kor J Hort Sci Technol 30(1):79–84
Acknowledgments
The authors are grateful to Jeanne A. T. Glória for the valuable technical assistance with histochemical analyses, to Thaís J. Vasconcellos for help in using the Image-Pro Plus software, and Adriana M. Lanziotti for lab assistance.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - (CAPES/Brazil) - Finance Code 001 - and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) through an international collaborative research project (Program Science without Borders - Project no. A054/2013) between the Núcleo de Biotecnologia Vegetal of the Universidade do Estado do Rio de Janeiro (UERJ/Brazil) and the Institut de Recherche pour le Développement (IRD/Montpellier, France). The work was also supported by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ/Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva Cordeiro, L., Collin, M., Callado, C.H. et al. Long-term conservation of Tarenaya rosea (Cleomaceae) root cultures: histological and histochemical analyses during cryopreservation using the encapsulation-vitrification technique. Protoplasma 257, 1021–1033 (2020). https://doi.org/10.1007/s00709-020-01486-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01486-0