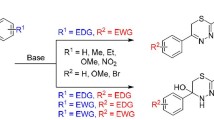
Summary.
Several novel N-(2,3-dihydro-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3-yl)-2-carboxamides were prepared by acyl coupling of 2-aminobenzophenones with α-(benzotriazol-1-yl)-N-acylglycines followed by displacement of the benzotriazole ring with ammonia and cyclization of the resulting monoacyl aminals. In addition to high yields and shorter reaction sequences due to avoiding deprotection and acylation of the protected 3-amino-1,4-benzodiazepin-2-one intermediates, the present approach did not involve the use of toxic and odoriferous materials as is the case with other methods.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received September 20, 2000. Accepted (revised) November 29, 2000
Rights and permissions
About this article
Cite this article
Khalaj, A., Pirali, M., Matloubi, H. et al. A Short and Efficient Synthesis of Novel N-(2,3-dihydro-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3-yl)-2-carboxamides. Monatshefte fuer Chemie 132, 747–752 (2001). https://doi.org/10.1007/s007060170090
Issue Date:
DOI: https://doi.org/10.1007/s007060170090