Abstract
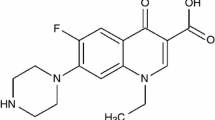
The synthesis of novel ternary M(II)/(III)/(IV) complexes with fluoroquinolone drug enrofloxacin (HEFX) and glycine (HGly) containing nitrogen and oxygen donor ligands are prepared and characterized. The prepared complexes have the general formulae of [M(EFX)(Gly)(H2O)2]Cl·xH2O (M = Cr(III), x = 0 and Fe(III), x = 1), [M(EFX)(Gly)(H2O)2]·xH2O (M = Mn(II), x = 0; Co(II), x = 0, Ni(II), x = 1; Cu(II), x = 2; and Zn(II), x = 0), [UO2(EFX)(Gly)]·3H2O, and [Th(EFX)(Gly)(H2O)2]Cl2. They are prepared and characterized based on elemental analysis, IR, 1H NMR, magnetic moment, molar conductance and thermal analyses (TG and DTA) techniques. The important bands in the IR spectra and main 1H NMR signals are tentatively assigned and discussed in relation to the predicted molecular structure. The IR data of the HEFX and HGly ligands suggested the existing of a bidentate binding involving carboxylate O and carbonyl for HEFX ligand and amino N and carboxylate O atoms for HGly ligand. The coordination geometries and electronic structures are determined from the diffused reflectance spectra and magnetic moment measurements. The complexes exist in octahedral form. The thermodynamic parameters, such as E*, ΔH*, ΔS*, and ΔG* are calculated from the TG curves using Coats-Redfern method. The HEFX drug, HGly, and the ternary metal complexes are also screened for their in vitro antimicrobial activity against bacterial (Escherichia coli and Staphylococcus aureus) and fungal (Aspergillus flavus and Candida albicans) organisms. The activity data show that HEFX drug and most of the metal complexes have bacterial activity more than the standard. Also the complexes have nearly comparable antifungal activity like that of the parent HEFX drug.
Graphical Abstract








Similar content being viewed by others
References
Lohray BB, Lohray VB, Srivastava BK, Kapadnis P, Pandya PP (2004) Bioorg Med Chem 12:4557
Beraldo H, Gambino D (2004) Mini-Rev Med Chem 4:31
Vieira LMM, De Almeida MV, De Abreu HA, Duarte HA, Grazul RM, Fontes APS (2009) Inorg Chim Acta 362:2060
Turel I (2002) Coord Chem Rev 232:27
Vieira MM, De Almeida MV, Lourene MCS, Bezerra OFAF, Fontes APS (2009) Eur J Med Chem 44:4107
Boothe DM (1994) Vet Med 89:744
Souza MJ, Bittene Court CF, Morsch IM (2002) Pharm Biomed Anal 28:1159
Saraiva R, Lopes S, Ferreira M, Novais F, Pereira F, Feio MJ, Gameiro P (2010) J Inorg Biochem 104:843
Efthimiadou EK, Katsaros N, Karaliota A, Psomas G (2007) Bioorg Med Chem Lett 17:1238
Turel I, Ledan I, Klintschar G, Bukovec N, Zalar S (1997) J Inorg Biochem 66:77
Mustafa JA (2002) Acta Chim Slov 49:457
Ruiz M, Perello L, Server-Carrio J, Ortiz R, Garcia-Granda S, Diaz MR, Canton E (1998) J Inorg Biochem 69:231
El-Gamal NEA, Mohamed RR, Zayed MA (2012) Dalton Trans 41:1824
Refat MS (2007) Spectrochim Acta A 68:1393
Refat MS, Mohamed GG, De Farias RF, Powell AK, El-Garib MS, El-Korashy SA, Hussien MA (2010) Therm Anal Calor 102:225
Geary WJ (1971) Coord Chem Rev 7:81
Sece HJ, Quiros M, Garmendia MJ (2000) Polyhedron 19:1005
Abd El-halim HF, Mohamed GG, El-Dessouky MMI, Mahmoud WH (2011) Spectrochim Acta A 82:8
Abd El-halim HF, Mohamed GG, El-Dessouky MMI, Mahmoud WH (2012) J Pharm Res 5:5084
Soliman MH, Hindy AMM, Mohammed GG (2014) Therm Anal Calorim 115:987
Gehad G, Mohamed GG, Hanan F, Abd El-Halim HF, Maher MI, El-Dessouky MMI, Walaa H, Mahmoud WH (2011) J Mol Str 999:29
Kumar S, Rai A, Rai SB, Ria DK, Singh VB (2005) Spectrochim Acta A 61:2741
Henrici-Olive G, Olive S (1984) The chemistry of the catalyzed hydrogenation of carbon monoxide. Springer, Berlin
Mohammed GG, Soliman AA (2004) Thermochim Acta 421:151
Sandhu GK, Verma SP (1987) Polyhedron 6:587
Aletras V, Hadjiliadis N, Lippert B (1992) Polyhedron 11:1359
Zidan AS, El-Said AI, El-Meligy MS, Aly AA, Mohamed OF (2000) J Therm Anal Calorim 26:665
Kumar AS, Rai A, Rai SB, Rai DK, Singh AN, Singh VB (2006) J Mol Str 791:23
Kumar S, Rai A, Rai SB, Rai DK, Singh VB (2005) Spectrochim Acta A 61:2741
Mohammed GG, Soliman MH (2010) Spectrochim Acta A 76:341
Soliman MH, Mohammed GG (2013) Spectrochim Acta A 107:5
Mohammed GG, Omar MM, Ibrahim AA (2009) Spectrochim Acta A 73:358
Mondal N, Dey DK, Mitra S, Abdul Malik KM (2000) Polyhedron 19:2707
Mohammed GG, Omar MM, Ahmed MM (2005) Spectrochim Acta A 62:1140
Yoshida MI, Gomes ECL, Soares CDV, Cunha AF, Oliveira MA (2010) Molecules 15:2439
Cotton FA, Wilkinson G, Murillo CA, Bochmann M (1999) Advanced inorganic chemistry, 6th edn. Wiley, New York
Coats AW, Redfern JP (1964) Nature 201:68
Ramesh R, Maheswaran S (2003) J Inorg Biochem 96:457
Thankamony M, Mohanan K (2007) Indian J Chem 46A:247
Bauer AW, Kirby WM, Sherris C, Turc KM (1966) Am J Clin Pathol 45:493
Pfaller MA, Burmeister L, Bartlett MA, Rinaldi MG (1988) J Clin Microbiol 26:1437
National Committee for Vlinical Laboratory Standands (2002) Reference Method for broth dilution antifungal susceptibility testing of conidium-forming filamentous: proposed standard M38-A. Wayne, PA
National Committee for Clinical Laboratory Standards (2003) Method for antifungal disc diffusion susceptibility testing of yeast: proposed guideline M 44-P. Wayne, PA
Liebowitz LD, Ashbee HR, Evans EG, Chong Y, Mallatova N, Zaidi M, Gibbs D (2001) J Microbiol Infect Dis 4:27
Matar MJ, Zeichner LO, Paetznik VL, Rodriguez JR, Chen E, Rex JH (2003) J Agents Chemother 47:1647
Acknowledgments
The authors wish to express their deep thank for Ms. Maha Magdy for carrying out the biological activity study in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soliman, M.H., Mohamed, G.G. & Hindy, A.M.M. Biological activity, spectral and thermal characterization of mixed ligand complexes of enrofloxacin and glycine: in vitro antibacterial and antifungal activity studies. Monatsh Chem 146, 259–273 (2015). https://doi.org/10.1007/s00706-014-1315-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1315-5