Abstract
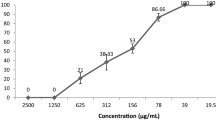
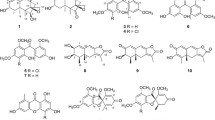
Seven natural products, including one new sesquiterpenoid (eudesm-1β, 6α, 11-triol, compound 1), one ergosta -4, 6, 8(14), 22-tetraen-3-one (compound 2), four polyphenols (compounds 3, 4, 5, 6), and one pyrone (3-hydroxy-2-methyl-4-pyrone, compound 7) were isolated from cultures of Phellinus ignarius by column chromatography. The detailed structure of compound 1 was determined using a combination of one- and two-dimensional nuclear magnetic resonance, mass spectrometry and infrared spectroscopy. The antiviral activity of these compounds against H5N1 influenza A virus was investigated using an MTT colorimetric assay system in Madin-Darby canine kidney cells. The results indicate that compound 1 possesses significant ability to inhibit influenza virus. The 50 % effective concentration was 0.14 ± 0.04 μM. Molecular modeling further suggested that the anti-influenza virus activity of this compound was partially attributed to the interactions of hydroxyl groups with an amino acid residue (Asn 170) of neuraminidase (NA) at the binding site. Moreover, the results of enzyme inhibition assays indicated that 50 % inhibition of NA was achieved by compound 1 at a concentration of 0.657 ± 0.325 mg/mL, which suggested that compound 1 is likely to interact with the NA enzyme.




Similar content being viewed by others
References
Cho HG, Choi JH, Kim WH, Hong HK, Yoon MH, Jho EH, Kang C, Lim YH (2012) High prevalence of amantadine-resistant influenza A virus isolated in Gyeonggi Province, South Korea, during 2005–2010. Arch Virol. doi:10.1007/s00705-012-1482-9
Yeh J-Y, Coumar MS, Horng J-T et al (2010) Anti-influenza drug discovery: structure-activity relationship and mechanistic insight into novel angelicin derivatives. J Med Chem 53:1519
Ge S, Zheng D, Zhao Y et al (2012) Evaluating viral interference between influenza virus and newcastle disease virus using real-time reverse transcription–polymerase chain reaction in chicken eggs. Virol J 9:128. doi:10.1186/1743-422X-9-128
Dermody TS, Sandri-Goldin RM, Shenk T (2013) A New Determinant of H5N1 Influenza Virus Pathogenesis in Mammals. J Virol. doi:10.1128/JVI.00474-13
Malakoff D, Enserink M (2013) Dual use research. New U.S. rules increase oversight of H5N1 studies, other risky science. Science 339(6123):1025
He P, Geng L, Wang J, Wang Z, Mao D, Xu C (2012) Purification, characterization and bioactivity of an extracellular polysaccharide produced from Phellinus igniarius. Ann Microbiol. doi:10.1007/s13213-012-0427-6
Wang G, Dong L, Zhang Y (2012) Polysaccharides from Phellinus linteus inhibit cell growth and invasion and induce apoptosis in HepG2 human hepatocellular carcinoma cells. Biologia 67:247
Dong W, Ning L et al (2009) Tumor-inhibitory and liver-protective effects of Phellinus igniarius extracellular polysaccharides. World J Microbiol Biotechnol 25(4):633
Zou X, Guo X, Sun M (2009) pH control strategy in a shaken minibioreactor for polysaccharide production by medicinal mushroom Phellinus linteus and its anti-hyperlipemia activity. Bioprocess Biosyst Eng 32:277
Wu X, Lin S, Zhu C et al (2010) Homo- and heptanor-sterols and tremulane sesquiterpenes from cultures of Phellinus igniarius. J Nat Prod 73:1294–1300
Wu X, Lin S, Zhu C et al (2010) Constituents of cultures of fungus Phellinus igniarius. China J Chin Materia Medica 36(7):874
Dao TT, Tung BT, Nguyen PH et al (2010) C-methylated flavonoids from cleistocalyx operculatus and their inhibitory effects on novel influenza A (H1N1) neuraminidase. J Nat Prod 73:1636
Motoki Takagi, Keiichiro Motohashi, Aya Nagai et al (2010) Anti-nfluenza Virus Compound from Streptomyces sp. RI18. ORGANIC LETTERS 12(20): 4664-4666
Shie JJ, Fang JM et al (2011) A practical synthesis of zanamivir phosphonate congeners with potent anti-influenza activity. J Am Chem Soc 133(44):17959
Das K (2012) Antivirals targeting influenza A virus. J Med Chem. doi:10.1021/jm300455c
Varghese JN, Laver WG, Colman PM (1983) Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 Å resolution. Nature 303:35–40
Russell RJ, Haire LF et al (2006) The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 443(7107):45
Bao-Kai Cui, Cony Decock (2012) Phellinus castanopsidis sp. nov. (Hymenochaetaceae) from southern China, with preliminary phylogeny based on rDNA sequences. Mycol Progress. doi:10.1007/s11557-012-0839-5
Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Epidemiol 27(3):493
Bing FH, Liu J et al (2009) Anti-influenza virus activity of total alkaloids from Commelina communis L. Arch Virol 154(11):1837
Kwon HJ, Kim HH, Yoon SY, Ryu YB et al (2010) In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol J 7:307
Wu QX, Shi YP, Jia ZJ (2006) Eudesmane sesquiterpenoids from the Asteraceae family. Nat Prod Rep 23:699
Ohmoto T, Ikeda K, Nomura S et al (1987) Studies on the sesquiterpenes from Ambrosia elatior LINNE. Chem Pharm Bull 35(6):2272
Iijima T, Yaoita Y, Kikuchi M (2003) Five new sesquiterpenoids and a new diterpenoid from Erigeron annuus (L.) PERS., Erigeron philadelphicus L. and Erigeron sumatrensis RETZ. Chem Pharm Bull 51(5):545
Zhao Y, Yue JM, He YN et al (1997) Eleven new eudesmane derivatives from Laggera pterodonta. J Nat Prod 60:545
Haruhiro F, Etsuko N, Emi O (2004) Six immunosuppressive features from an scomycete, Zopfiella longicaudata, found in a screening study monitored by immunomodulatory activity. Chem Pharm Bull 52(8):1005
Zhu X, Da S, Huan L (2007) Polyphenols based on isoflavones as inhibitors of Helicobacter pylori urease. Bioorg Med Chem 15:3703–3710
Aldrich Library of 13C and 1H FT NMR Spectra (1992) 2: 180C
Aldrich Library of 13C and 1H FT NMR Spectra (1992) 2: 276C, 296A
Ronald B, Bhatia P (1982) Catechol O-Methyltransferase. 12. Affinity labeling the active site with the oxidation products of 5, 6-Dihydroxyindole. J Med Chem 25:263–271
Aldrich Library of 13C and 1H FT NMR Spectra (1992) 1:724A
Acknowledgments
This project was financially supported by Science and Technology Development Plan of Shandong Province (No. 2010GNC10956). We are grateful for the help of Professor Ji-Kai Liu. We thank Dr. Martin Sadilek and Loren Kruse (Department of Chemistry, University of Washington, USA) for English editing. We also thank BioBioPha Co., Ltd. and National Reference Laboratory for Newcastle Disease, China Animal Health and Epidemiology Center for their contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
A.-R. Song, X.-L. Sun, and C. Kong equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, AR., Sun, XL., Kong, C. et al. Discovery of a new sesquiterpenoid from Phellinus ignarius with antiviral activity against influenza virus. Arch Virol 159, 753–760 (2014). https://doi.org/10.1007/s00705-013-1857-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-013-1857-6