Abstract
Extrapyramidal movement disorders comprise hypokinetic-rigid and hyperkinetic or mixed forms, most of them originating from dysfunction of the basal ganglia (BG) and their information circuits that have been briefly reviewed in part 1 of the papers on neuropathology and pathogenesis of extrapyramidal movement disorders. The classification of hyperkinetic forms distinguishes the following: (1) chorea and related syndromes; (2) dystonias (dyskinesias); (3) tics and tourette disorders; (4) ballism; (5) myoclonic and startle disorders; and (6) tremor syndromes. Recent genetic and molecular classification distinguishes the following: (1) polyglutamine disorders (Huntington’s disease and related disorders); (2) pantothenate kinase associated neurodegeneration; (3) Wilson’s disease and related disorders; and (4) other hereditary neurodegenerations without hitherto detected genetic or specific markers. The diversity of phenotypes is related to the deposition of pathologic proteins in distinct cell populations, causing neurodegeneration due to genetic and environmental factors, but there is frequent overlap between various disorders. Their etiopathogenesis is still poorly understood but is suggested to result from an interaction between genetic and environmental factors, multiple etiologies, and noxious factors (protein mishandling, mitochondrial dysfunction, oxidative stress, excitotoxicity, energy failure, chronic neuroinflammation), being more likely than one single factor. Current clinical consensus criteria have increased the diagnostic accuracy of most neurodegenerative movement disorders, but for their definite diagnosis, histopathological confirmation is required. A timely overview of the neuropathology and pathogenesis of the major hyperkinetic movement disorders is presented.

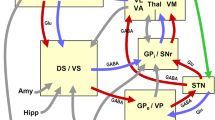
From (Jellinger 2016b)
Similar content being viewed by others
Abbreviations
- α-Syn:
-
α-Synuclein
- AD:
-
Alzheimer's disease
- AGs:
-
Argyrophilic grains
- ALS:
-
Amyptrophic lateral sclerosis
- ATN1:
-
Atrophin-1
- AutD:
-
Autosomal-dominant
- AutR:
-
Autosomal-recessive
- BDNF:
-
Brain-derived neurotrophic factor
- BG:
-
Basal ganglia
- BHC:
-
Benign hereditary chorea
- BIBD:
-
Basophilic inclusion body disease
- CAA:
-
Cerebral amyloid angiopathy
- CAG:
-
Polyglutamine
- CBD:
-
Corticobasal degeneration
- CBGTC:
-
Cortico-BG-thalamocortical
- CBS:
-
Corticobasal syndrome
- CD:
-
Cervical dystonia
- ChAc:
-
Chorea-acanthocytosis
- ChAT:
-
Choline-acetyl transferase
- CI:
-
Cognitive impairment
- CN:
-
Caudate nucleus
- CNS:
-
Central nervous system
- CoA:
-
Coenzyme A
- DA:
-
Dopamine
- DLB:
-
Dementia with Lewy bodies
- DRD:
-
Dopa-responsive dystonia
- DRPLA:
-
Dentatorubral–pallidoluysian atrophy
- DS:
-
Dystonia syndrome
- ENK:
-
Enkephalin
- ET:
-
Essential tremor
- FTL:
-
Ferritin light chain
- FUS:
-
Fused-in sarcoma
- GABA:
-
γ-aminobutyric acid
- GBA:
-
Glucocerebrosidase gene
- GCase:
-
Glucocerebrosidase
- GDNF:
-
Glia-derived neurotrophic factor
- GPe:
-
External segment of globus pallidus
- GPi:
-
Internal segment of globus pallidus
- HD:
-
Huntington’s disease
- HDL:
-
Huntington disease-like
- HTT:
-
Huntingtin
- LaBs:
-
Lafora bodies
- LB:
-
Lewy body
- LC:
-
Locus ceruleus
- MCI:
-
Mild cognitive impairment
- MD:
-
Menkes’ disease
- mHTT:
-
Mutant huntingtin
- MJD:
-
Machado-Joseph disease
- MRI:
-
Magnetic resonance imaging
- MSA:
-
Multiple system atrophy
- MSN:
-
Medium spiny projection neuron
- NA:
-
Neuroacanthocytosis
- NBIA:
-
Neurodegeneration with brain iron accumulation
- NBM:
-
Nucleus basalis of Meynert
- NFTs:
-
Neurofibrillary tangles
- NIID:
-
Neuronal intranuclear inclusion disease
- NT:
-
Neuropil threads
- OCD:
-
Obsessive-compulsive disorder
- OS:
-
Oxidative stress
- PC:
-
Purkinje cell
- PD:
-
Parkinson’s disease
- PDC:
-
Parkinson’s disease complex
- PKAN:
-
Pantothenate-kinase associated neurodegeneration
- PKD:
-
Paroxysmal kinesic dyskinesia
- PLAN:
-
Phospholipase A2-associated neurodegeneration
- PNKD:
-
Paroxysmal non-kinesic dyskinesias
- PPN:
-
pedunculopontine nucleus
- PPT:
-
Pedunculo-pontine tegmental
- PSP:
-
Progressive supranuclear palsy
- Put:
-
Putamen
- RDP:
-
Rapid-onset dystonia-parkinsonism
- SCA:
-
Spinocerebellar ataxia
- SN:
-
Substantia nigra
- SNc:
-
Substantia nigra pars compacta
- SNr:
-
Substantia nigra pars reticulata
- SP:
-
Substance P
- STN:
-
Subthalamic nucleus
- TDP-43:
-
Transactive DNA-binding protein
- TS:
-
Tourette's syndrome
- Ub:
-
Ubiquitin
- VM:
-
Ventromedial
- VTA:
-
Ventral tegmental area
- WD:
-
Wilson’s disease
- XDP:
-
X-linked dystonia-parkinsonism
References
Agarwal S, Biagioni MC (2019) Essential Tremor, 2018/05/16 edition. StatPearls Publishing, Treasure Island
Albanese A (2017) How many dystonias? Clinical evidence. Front Neurol 8:18
Albanese A, Sorbo FD (2016) Dystonia and tremor: the clinical syndromes with isolated tremor. Tremor Other Hyperkinet Mov (N Y) 6:319
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord 28:863–873
Albanese A, Di Giovanni M, Lalli S (2019) Dystonia: diagnosis and management. Eur J Neurol 26:5–17
Albin RL, Mink JW (2006) Recent advances in Tourette syndrome research. Trends Neurosci 29:175–182
Aldaz T, Nigro P, Sanchez-Gomez A, Painous C, Planellas L, Santacruz P, Camara A, Compta Y, Valldeoriola F, Marti MJ, Munoz E (2019) Non-motor symptoms in Huntington’s disease: a comparative study with Parkinson’s disease. J Neurol 266:1340–1350
Alvarez-Cordoba M, Fernandez Khoury A, Villanueva-Paz M, Gomez-Navarro C, Villalon-Garcia I, Suarez-Rivero JM, Povea-Cabello S, de la Mata M, Cotan D, Talaveron-Rey M, Perez-Pulido AJ, Salas JJ, Perez-Villegas EM, Diaz-Quintana A, Armengol JA, Sanchez-Alcazar JA (2019a) Pantothenate rescues iron accumulation in pantothenate kinase-associated neurodegeneration depending on the type of mutation. Mol Neurobiol 56:3638–3656
Alvarez-Cordoba M, Villanueva-Paz M, Villalon-Garcia I, Povea-Cabello S, Suarez-Rivero JM, Talaveron-Rey M, Abril-Jaramillo J, Vintimilla-Tosi AB, Sanchez-Alcazar JA (2019b) Precision medicine in pantothenate kinase-associated neurodegeneration. Neural Regen Res 14:1177–1185
Anderson DG, Walker RH, Connor M, Carr J, Margolis RL, Krause A (2017) A systematic review of the Huntington disease-like 2 phenotype. J Huntingt Dis 6:37–46
Anderson DG, Ferreira-Correia A, Rodrigues FB, Aziz NA, Carr J, Wild EJ, Margolis RL, Krause A (2019a) Comparison of the Huntington’s disease like 2 and Huntington’s disease clinical phenotypes. Mov Disord Clin Pract 6:302–311
Anderson DG, Haagensen M, Ferreira-Correia A, Pierson R, Carr J, Krause A, Margolis RL (2019b) Emerging differences between Huntington’s disease-like 2 and Huntington’s disease: a comparison using MRI brain volumetry. Neuroimage Clin 21:101666
Aneichyk T, Hendriks WT, Yadav R, Shin D, Gao D, Vaine CA, Collins RL, Domingo A, Currall B, Stortchevoi A, Multhaupt-Buell T, Penney EB, Cruz L, Dhakal J, Brand H, Hanscom C, Antolik C, Dy M, Ragavendran A, Underwood J, Cantsilieris S, Munson KM, Eichler EE, Acuna P, Go C, Jamora RDG, Rosales RL, Church DM, Williams SR, Garcia S, Klein C, Muller U, Wilhelmsen KC, Timmers HTM, Sapir Y, Wainger BJ, Henderson D, Ito N, Weisenfeld N, Jaffe D, Sharma N, Breakefield XO, Ozelius LJ, Bragg DC, Talkowski ME (2018) Dissecting the causal mechanism of X-linked dystonia-parkinsonism by integrating genome and transcriptome assembly. Cell 172(897–909):e821
Aridon P, Tarantino P, Ragonese P, D’Amelio M, Cinturino A, Salemi G, Gagliardi M, Lo Re V, Scarpitta A, Gambardella A, Quattrone A, Annesi G, Savettieri G (2012) Dentatorubral–pallidoluysian atrophy: haplotype of Asian origin in 2 Italian families. Mov Disord 27:460–461
Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431:805–810
Augustine F, Singer HS (2019) Merging the pathophysiology and pharmacotherapy of tics. Tremor Other Hyperkinet Mov (N Y) 8:595
Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, Lyons KE, Faust PL, Vonsattel JP (2008) Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol 65:101–107
Baide-Mairena H, Gaudo P, Marti-Sanchez L, Emperador S, Sanchez-Montanez A, Alonso-Luengo O, Correa M, Grau AM, Ortigoza-Escobar JD, Artuch R, Vazquez E, Del Toro M, Garrido-Perez N, Ruiz-Pesini E, Montoya J, Bayona-Bafaluy MP, Perez-Duenas B (2019) Mutations in the mitochondrial complex I assembly factor NDUFAF6 cause isolated bilateral striatal necrosis and progressive dystonia in childhood. Mol Genet Metab 126:250–258
Baig SS, Strong M, Quarrell OW (2016) The global prevalence of Huntington’s disease: a systematic review and discussion. Neurodegener Dis Manag 6:331–343
Baizabal-Carvallo JF, Hallett M, Jankovic J (2019) Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis 127:32–44
Balint B, Bhatia KP (2015) Isolated and combined dystonia syndromes—an update on new genes and their phenotypes. Eur J Neurol 22:610–617
Balint B, Vincent A, Meinck HM, Irani SR, Bhatia KP (2018) Movement disorders with neuronal antibodies: syndromic approach, genetic parallels and pathophysiology. Brain 141:13–36
Balint B, Charlesworth G, Stamelou M, Carr L, Mencacci NE, Wood NW, Bhatia KP (2019) Mitochondrial complex I NUBPL mutations cause combined dystonia with bilateral striatal necrosis and cerebellar atrophy. Eur J Neurol. https://doi.org/10.1111/ene.13956
Bansagi B, Lewis-Smith D, Pal E, Duff J, Griffin H, Pyle A, Muller JS, Rudas G, Aranyi Z, Lochmuller H, Chinnery PF, Horvath R (2016) Phenotypic convergence of Menkes and Wilson disease. Neurol Genet 2:e119
Baskota SU, Lopez OL, Greenamyre JT, Kofler J (2018) Spectrum of tau pathologies in Huntington’s disease. Lab Invest. https://doi.org/10.1038/s41374-41018-40166-41379
Begeti F, Schwab LC, Mason SL, Barker RA (2016) Hippocampal dysfunction defines disease onset in Huntington’s disease. J Neurol Neurosurg Psychiatry 87:975–981
Beier K, Pratt DP (2019) Chorea, Sydenham, 2017/06/15 edition. StatPearls Publishing, Treasure Island
Beliveau E, Tremblay C, Aubry-Lafontaine E, Paris-Robidas S, Delay C, Robinson C, Ferguson L, Rajput AH, Rajput A, Calon F (2015) Accumulation of amyloid-beta in the cerebellar cortex of essential tremor patients. Neurobiol Dis 82:397–408
Berman BD, Junker J, Shelton E, Sillau SH, Jinnah HA, Perlmutter JS, Espay AJ, Jankovic J, Vidailhet M, Bonnet C, Ondo W, Malaty IA, Rodriguez R, McDonald WM, Marsh L, Zurowski M, Baumer T, Bruggemann N (2017) Psychiatric associations of adult-onset focal dystonia phenotypes. J Neurol Neurosurg Psychiatry 88:595–602
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, Raethjen J, Stamelou M, Testa CM, Deuschl G (2018) Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 33:75–87
Bianchi S, Fuertinger S, Huddleston H, Frucht SJ, Simonyan K (2019) Functional and structural neural bases of task specificity in isolated focal dystonia. Mov Disord 34:555–563
Blood AJ, Kuster JK, Waugh JL, Levenstein JM, Multhaupt-Buell TJ, Sudarsky LR, Breiter HC, Sharma N (2019) White matter changes in cervical dystonia relate to clinical effectiveness of botulinum toxin treatment. Front Neurol 10:265
Bohlega SA, Abou-Al-Shaar H, AlDakheel A, Alajlan H, Bohlega BS, Meyer BF, Monies D, Cupler EJ, Al-Saif AM (2019) Autosomal recessive ADCY5-related dystonia and myoclonus: expanding the genetic spectrum of ADCY5-related movement disorders. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2019.1002.1039
Bokhari MR, Bokhari SRA (2018) Hallervorden Spatz disease (pantothenate kinase-associated neurodegeneration, PKAN), 2017/06/15 Edition. StatPearls Publishing, Treasure Island
Bologna M, Berardelli A (2018) The cerebellum and dystonia. Handb Clin Neurol 155:259–272
Bostantjopoulou S, Stathis P, Konitsiotis S (2017) Wilson’s disease: neurological aspects, clinical manifestations, and treatment considerations. In: Falup-Pecurariu C, Ferreira J, Martinez-Martin P, Chaudhuri KR (eds) Movement disorders curricula. Springer, Wien, pp 343–354
Bragg DC, Mangkalaphiban K, Vaine CA, Kulkarni NJ, Shin D, Yadav R, Dhakal J, Ton ML, Cheng A, Russo CT, Ang M, Acuna P, Go C, Franceour TN, Multhaupt-Buell T, Ito N, Muller U, Hendriks WT, Breakefield XO, Sharma N, Ozelius LJ (2017) Disease onset in X-linked dystonia-parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1. Proc Natl Acad Sci USA 114:E11020–E11028
Bragg DC, Sharma N, Ozelius LJ (2019) X-linked dystonia-parkinsonism: recent advances. Curr Opin Neurol. https://doi.org/10.1097/WCO.0000000000000708
Brander G, Isomura K, Chang Z, Kuja-Halkola R, Almqvist C, Larsson H, Mataix-Cols D, Fernandez de la Cruz L (2019) Association of Tourette syndrome and chronic tic disorder with metabolic and cardiovascular disorders. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2018.4279
Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG (2008) The pathophysiological basis of dystonias. Nat Rev Neurosci 9:222–234
Bruggemann N, Heldmann M, Klein C, Domingo A, Rasche D, Tronnier V, Rosales RL, Jamora RD, Lee LV, Munte TF (2016) Neuroanatomical changes extend beyond striatal atrophy in X-linked dystonia parkinsonism. Parkinsonism Relat Disord 31:91–97
Bruzelius E, Scarpa J, Zhao Y, Basu S, Faghmous JH, Baum A (2019) Huntington’s disease in the United States: variation by demographic and socioeconomic factors. Mov Disord 34:858–865
Buijink AW, van der Stouwe AM, Broersma M, Sharifi S, Groot PF, Speelman JD, Maurits NM, van Rootselaar AF (2015) Motor network disruption in essential tremor: a functional and effective connectivity study. Brain 138:2934–2947
Cadenas B, Fita-Torro J, Bermudez-Cortes M, Hernandez-Rodriguez I, Fuster JL, Llinares ME, Galera AM, Romero JL, Perez-Montero S, Tornador C, Sanchez M (2019) l-Ferritin: one gene, five diseases; from hereditary hyperferritinemia to hypoferritinemia-report of new cases. Pharmaceuticals (Basel) 12:17
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Caligiore D, Mannella F, Arbib MA, Baldassarre G (2017) Dysfunctions of the basal ganglia-cerebellar-thalamo-cortical system produce motor tics in Tourette syndrome. PLoS Comput Biol 13:e1005395
Caputi C, Tolve M, Galosi S, Inghilleri M, Carducci C, Angeloni A, Leuzzi V (2019) PNKP deficiency mimicking a benign hereditary chorea: the misleading presentation of a neurodegenerative disorder. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2019.1003.1012
Cardaioli G, Ripandelli F, Paolini Paoletti F, Nigro P, Simoni S, Brahimi E, Romoli M, Filidei M, Eusebi P, Calabresi P, Tambasco N (2019) Substantia nigra hyperechogenicity in essential tremor and Parkinson disease: a longitudinal study. Eur J Neurol. https://doi.org/10.1111/ene.13988
Cardoso F (2014) Differential diagnosis of Huntington’s disease: what the clinician should know. Neurodegener Dis Manag 4:67–72
Cardoso F (2017a) Autoimmune choreas. J Neurol Neurosurg Psychiatry 88:412–417
Cardoso F (2017b) Nonmotor symptoms in Huntington disease. Int Rev Neurobiol 134:1397–1408
Carroll LS, Massey TH, Wardle M, Peall KJ (2018) Dentatorubral–pallidoluysian atrophy: an update. Tremor Other Hyperkinet Mov (N Y) 8:577
Catai LMP, Camargo CHF, Moro A, Ribas G, Raskin S, Teive HAG (2018) Dystonia in patients with spinocerebellar ataxia 3—Machado–Joseph disease: an underestimated diagnosis? Open Neurol J 12:41–49
Chan CS, Surmeier DJ (2014) Astrocytes go awry in Huntington’s disease. Nat Neurosci 17:641–642
Chaudhry HS, Anilkumar AC (2019) Wilson disease, 2017/07/20 edition. StatPearls Publishing, Treasure Island
Chen S, Gan SR, Cai PP, Ni W, Zhou Q, Dong Y, Wang N, Wu ZY (2016) Mitochondrial NADH dehydrogenase subunit 3 polymorphism associated with an earlier age at onset in male Machado–Joseph disease patients. CNS Neurosci Ther 22:38–42
Chinnery PF (2018) Neuroferritinopathy. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews® [Internet], 2018/01/18 edition. University of Washington, Seattle. http://www.ncbi.nlm.nih.gov/books/NBK1141. Accessed 3 June 2019
Choe M, Cortes E, Vonsattel JP, Kuo SH, Faust PL, Louis ED (2016) Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord 31:393–401
Cirillo G, Cirillo M, Panetsos F, Virtuoso A, Papa M (2019) Selective vulnerability of basal ganglia: insights into the mechanisms of bilateral striatal necrosis. J Neuropathol Exp Neurol 78:123–129
Connolly BS, Hazrati LN, Lang AE (2014) Neuropathological findings in chorea-acanthocytosis: new insights into mechanisms underlying parkinsonism and seizures. Acta Neuropathol 127:613–615
Conte A, Defazio G, Hallett M, Fabbrini G, Berardelli A (2019) The role of sensory information in the pathophysiology of focal dystonias. Nat Rev Neurol 15:224–233
Contessotto MGG, Rosselli-Murai LK, Garcia MCC, Oliveira CLP, Torriani IL, Lopes-Cendes I, Murai MJ (2018) The Machado–Joseph disease-associated expanded form of ataxin-3: overexpression, purification, and preliminary biophysical and structural characterization. Protein Expr Purif 152:40–45
Cortes CJ, La Spada AR (2019) TFEB dysregulation as a driver of autophagy dysfunction in neurodegenerative disease: Molecular mechanisms, cellular processes, and emerging therapeutic opportunities. Neurobiol Dis 122:83–93
Corp DT, Joutsa J, Darby RR, Delnooz CCS, van de Warrenburg BPC, Cooke D, Prudente CN, Ren J, Reich MM, Batla A, Bhatia KP, Jinnah HA, Liu H, Fox MD (2019) Network localization of cervical dystonia based on causal brain lesions. Brain 142:1660–1674
Croce KR, Yamamoto A (2019) A role for autophagy in Huntington’s disease. Neurobiol Dis 122:16–22
Cronin T, Rosser A, Massey T (2019) Clinical presentation and features of juvenile-onset Huntington’s disease: a systematic review. J Huntingt Dis 8:171–179
Dale RC (2017) Tics and Tourette: a clinical, pathophysiological and etiological review. Curr Opin Pediatr 29:665–673
Dafsari HS, Sprute R, Wunderlich G, Daimaguler HS, Karaca E, Contreras A, Becker K, Schulze-Rhonhof M, Kiening K, Karakulak T, Kloss M, Horn A, Pauls A, Nurnberg P, Altmuller J, Thiele H, Assmann B, Koy A, Cirak S (2019) Novel mutations in KMT2B offer pathophysiological insights into childhood-onset progressive dystonia. J Hum Genet. https://doi.org/10.1038/s10038-019-0625-1
Davis MY, Keene CD, Jayadev S, Bird T (2014) The co-occurrence of Alzheimer’s disease and Huntington’s disease: a neuropathological study of 15 elderly Huntington’s disease subjects. J Huntingt Dis 3:209–217
de Azevedo PC, Guimaraes RP, Piccinin CC, Piovesana LG, Campos LS, Zuiani JR, Tamashiro EM, Pinheiro G, Amato-Filho AC, Cendes F, Lopes-Cendes I, D’Abreu A (2017) Cerebellar gray matter alterations in Huntington disease: a voxel-based morphometry study. Cerebellum 16:923–928
Degirmenci N, Veyseller B, Hanagasi H, Bilgic B, Gurbuz D, Toprak A, Ozturan O (2019) Olfactory function and olfactory bulb volume in Wilson’s disease. Eur Arch Otorhinolaryngol 276:139–142
den Dunnen WF (2013) Neuropathological diagnostic considerations in hyperkinetic movement disorders. Front Neurol 4:7
Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A (2004) Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. J Chem Neuroanat 27:143–164
Desdentado L, Espert R, Sanz P, Tirapu-Ustarroz J (2019) Lafora disease: a review of the literature. Rev Neurol 68:66–74
DeSimone JC, Archer DB, Vaillancourt DE, Wagle Shukla A (2019) Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain 142:1644–1659
Di Meo I, Carecchio M, Tiranti V (2019) Inborn errors of coenzyme A metabolism and neurodegeneration. J Inherit Metab Dis 42:49–56
Ding D, Wang C, Chen Z, Peng H, Li K, Zhou X, Peng Y, Wang P, Hou X, Li T, Qiu R, Xia K, Sequeiros J, Ashizawa T, Tang B, Jiang H (2019) Polymorphisms in DNA methylation-related genes are linked to the phenotype of Machado-Joseph disease. Neurobiol Aging 75:225 e221–225 e228
Douglas AG, Andreoletti G, Talbot K, Hammans SR, Singh J, Whitney A, Ennis S, Foulds NC (2017) ADCY5-related dyskinesia presenting as familial myoclonus-dystonia. Neurogenetics 18:111–117
Duda JE, Jellinger KA (2011) Neurodegeneration with brain iron accumulation. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 446–455
Dusek P, Bahn E, Litwin T, Jablonka-Salach K, Luciuk A, Huelnhagen T, Madai VI, Dieringer MA, Bulska E, Knauth M, Niendorf T, Sobesky J, Paul F, Schneider SA, Czlonkowska A, Bruck W, Wegner C, Wuerfel J (2017) Brain iron accumulation in Wilson disease: a post mortem 7 Tesla MRI—histopathological study. Neuropathol Appl Neurobiol 43:514–532
Dusek P, Litwin T, Czlonkowska A (2019) Neurologic impairment in Wilson disease. Ann Transl Med 7:S64
Dziewulska D, Doi H, Fasano A, Erro R, Fatehi F, Fekete R, Gatto EM, Pablos EG, Lehn A, Miyajima H, Piperno A, Pellechia MT, Wu YR, Yoshida K, Zarruk JG, Jingli S, Schrag A, McNeill A (2013) Olfactory impairment and pathology in neurodegenerative disorders with brain iron accumulation. Acta Neuropathol 126:151–153
Edwards MJ, Stamelou M, Quinn N, Bhatia KP (2016) Parkinson’s disease and other movement disorders, 2nd edn. Oxford University Press, Oxford
Elble RJ (2013) Tremor disorders. Curr Opin Neurol 26:413–419
Elias WJ, Shah BB (2014) Tremor. JAMA 311:948–954
Erro R, Bhatia KP (2019) Unravelling of the paroxysmal dyskinesias. J Neurol Neurosurg Psychiatry 90:227–234
Eskow Jaunarajs KL, Bonsi P, Chesselet MF, Standaert DG, Pisani A (2015) Striatal cholinergic dysfunction as a unifying theme in the pathophysiology of dystonia. Prog Neurobiol 127–128:91–107
Farrell K, Cosentino S, Iida MA, Chapman S, Bennett DA, Faust PL, Louis ED, Crary JF (2019) Quantitative assessment of pathological tau burden in essential tremor: a postmortem study. J Neuropathol Exp Neurol 78:31–37
Fernandez-Nogales M, Cabrera JR, Santos-Galindo M, Hoozemans JJ, Ferrer I, Rozemuller AJ, Hernandez F, Avila J, Lucas JJ (2014) Huntington’s disease is a four-repeat tauopathy with tau nuclear rods. Nat Med 20:881–885
Ferrari Bardile C, Garcia-Miralles M, Caron NS, Rayan NA, Langley SR, Harmston N, Rondelli AM, Teo RTY, Waltl S, Anderson LM, Bae HG, Jung S, Williams A, Prabhakar S, Petretto E, Hayden MR, Pouladi MA (2019) Intrinsic mutant HTT-mediated defects in oligodendroglia cause myelination deficits and behavioral abnormalities in Huntington disease. Proc Natl Acad Sci U S A 116:9622–9627
Forde NJ, Zwiers MP, Naaijen J, Akkermans SEA, Openneer TJC, Visscher F, Dietrich A, Buitelaar JK, Hoekstra PJ (2017) Basal ganglia structure in Tourette’s disorder and/or attention-deficit/hyperactivity disorder. Mov Disord 32:601–604
Fu Z, Liu F, Liu C, Jin B, Jiang Y, Tang M, Qi X, Guo X (2019) Mutant huntingtin inhibits the mitochondrial unfolded protein response by impairing ABCB10 mRNA stability. Biochim Biophys Acta Mol Basis Dis 1865:1428–1435
Gallea C, Popa T, Garcia-Lorenzo D, Valabregue R, Legrand AP, Marais L, Degos B, Hubsch C, Fernandez-Vidal S, Bardinet E, Roze E, Lehericy S, Vidailhet M, Meunier S (2015) Intrinsic signature of essential tremor in the cerebello-frontal network. Brain 138:2920–2933
Gallea C, Popa T, Garcia-Lorenzo D, Valabregue R, Legrand AP, Apartis E, Marais L, Degos B, Hubsch C, Fernandez-Vidal S, Bardinet E, Roze E, Lehericy S, Meunier S, Vidailhet M (2016) Orthostatic tremor: a cerebellar pathology? Brain 139:2182–2197
Galstyan A, Wilbur C, Selby K, Hukin J (2017) Opsoclonus-myoclonus syndrome: a new era of improved prognosis? Pediatr Neurol 72:65–69
Ganos C (2016) Tics and Tourette’s: update on pathophysiology and tic control. Curr Opin Neurol 29:513–518
Ganos C, Kassavetis P, Erro R, Edwards MJ, Rothwell J, Bhatia KP (2014) The role of the cerebellum in the pathogenesis of cortical myoclonus. Mov Disord 29:437–443
Gao J, Brackley S, Mann JP (2019) The global prevalence of Wilson disease from next-generation sequencing data. Genet Med 21:1155–1163
Garcia-Gimeno MA, Knecht E, Sanz P (2018) Lafora disease: a ubiquitination-related pathology. Cells 7:87
Gardiner AR, Jaffer F, Dale RC, Labrum R, Erro R, Meyer E, Xiromerisiou G, Stamelou M, Walker M, Kullmann D, Warner T, Jarman P, Hanna M, Kurian MA, Bhatia KP, Houlden H (2015) The clinical and genetic heterogeneity of paroxysmal dyskinesias. Brain 138:3567–3580
Gelpi E, Llado A, Clarimon J, Rey MJ, Rivera RM, Ezquerra M, Antonell A, Navarro-Otano J, Ribalta T, Pinol-Ripoll G, Perez A, Valldeoriola F, Ferrer I (2012) Phenotypic variability within the inclusion body spectrum of basophilic inclusion body disease and neuronal intermediate filament inclusion disease in frontotemporal lobar degenerations with FUS-positive inclusions. J Neuropathol Exp Neurol 71:795–805
Gonzalez-Alegre P (2019) Advances in molecular and cell biology of dystonia: focus on torsinA. Neurobiol Dis 127:233–241
Govert F, Schneider SA (2013) Huntington’s disease and Huntington’s disease-like syndromes: an overview. Curr Opin Neurol 26:420–427
Gratuze M, Cisbani G, Cicchetti F, Planel E (2016) Is Huntington’s disease a tauopathy? Brain 139:1014–1025
Guo Z, Rudow G, Pletnikova O, Codispoti KE, Orr BA, Crain BJ, Duan W, Margolis RL, Rosenblatt A, Ross CA, Troncoso JC (2012) Striatal neuronal loss correlates with clinical motor impairment in Huntington’s disease. Mov Disord 27:1379–1386
Haggstrom L, Darveniza P, Tisch S (2017) Mild parkinsonian features in dystonia: literature review, mechanisms and clinical perspectives. Parkinsonism Relat Disord 35:1–7
Hall DA, Berry-Kravis E (2018) Fragile X syndrome and fragile X-associated tremor ataxia syndrome. Handb Clin Neurol 147:377–391
Hanspal E, Frucht S (2011) Myoclonus. In: Gálvez-Jiménez N, Tuite P (eds) Uncommon causes of movement disorders, 1st edn. Cambridge University Press, Cambridge, pp 175–179
Hanssen H, Heldmann M, Prasuhn J, Tronnier V, Rasche D, Diesta CC, Domingo A, Rosales RL, Jamora RD, Klein C, Munte TF, Bruggemann N (2018) Basal ganglia and cerebellar pathology in X-linked dystonia-parkinsonism. Brain 141:2995–3008
Haraguchi T, Terada S, Ishizu H, Yokota O, Yoshida H, Takeda N, Kishimoto Y, Katayama N, Takata H, Akagi M, Kuroda S, Ihara Y, Uchitomi Y (2011) Coexistence of TDP-43 and tau pathology in neurodegeneration with brain iron accumulation type 1 (NBIA-1, formerly Hallervorden–Spatz syndrome). Neuropathology 31:531–539
Hasegawa A, Ikeuchi T, Koike R, Matsubara N, Tsuchiya M, Nozaki H, Homma A, Idezuka J, Nishizawa M, Onodera O (2010) Long-term disability and prognosis in dentatorubral–pallidoluysian atrophy: a correlation with CAG repeat length. Mov Disord 25:1694–1700
Hayflick SJ, Kurian MA, Hogarth P (2018) Neurodegeneration with brain iron accumulation. Handb Clin Neurol 147:293–305
He R, Yan X, Guo J, Xu Q, Tang B, Sun Q (2018) Recent advances in biomarkers for Parkinson’s disease. Front Aging Neurosci 10:305
Hedera P (2019) Wilson’s disease: a master of disguise. Parkinsonism Relat Disord 59:140–145
Heimer G, Gregory A, Hogarth P, Hayflick S, Ben Zeev B (2019) MECR-related neurologic disorder. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews® [Internet], 2019/05/10 edition. University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK540959. Accessed 3 June 2019
Hobbs NZ, Pedrick AV, Say MJ, Frost C, Dar Santos R, Coleman A, Sturrock A, Craufurd D, Stout JC, Leavitt BR, Barnes J, Tabrizi SJ, Scahill RI (2011) The structural involvement of the cingulate cortex in premanifest and early Huntington’s disease. Mov Disord 26:1684–1690
Holton JL, Schneider SA, Ganesharajah T, Gandhi S, Strand C, Shashidharan P, Barreto J, Wood NW, Lees AJ, Bhatia KP, Revesz T (2008) Neuropathology of primary adult-onset dystonia. Neurology 70:695–699
Homayoon N, Pirpamer L, Franthal S, Katschnig-Winter P, Kogl M, Seiler S, Wenzel K, Hofer E, Deutschmann H, Fazekas F, Langkammer C, Ropele S, Schmidt R, Schwingenschuh P (2019) Nigral iron deposition in common tremor disorders. Mov Disord 34:129–132
Hopfner F, Deuschl G (2018) Is essential tremor a single entity? Eur J Neurol 25:71–82
Hopfner F, Haubenberger D, Galpern WR, Gwinn K, Van’t Veer A, White S, Bhatia K, Adler CH, Eidelberg D, Ondo W, Stebbins GT, Tanner CM, Helmich RC, Lenz FA, Sillitoe RV, Vaillancourt D, Vitek JL, Louis ED, Shill HA, Frosch MP, Foroud T, Kuhlenbaumer G, Singleton A, Testa CM, Hallett M, Elble R, Deuschl G (2016) Knowledge gaps and research recommendations for essential tremor. Parkinsonism Relat Disord 33:27–35
Hor CHH, Tang BL (2018) Beta-propeller protein-associated neurodegeneration (BPAN) as a genetically simple model of multifaceted neuropathology resulting from defects in autophagy. Rev Neurosci 30:261–277
Hwang J, Bank AM, Mortazavi F, Oakley DH, Frosch MP, Schmahmann JD (2019) Spinal cord a-synuclein deposition associated with myoclonus in patients with MSA-C. Neurology (accepted)
Iacono D, Geraci-Erck M, Peng H, Rabin ML, Kurlan R (2018) Hypertrophy of nigral neurons in Torsin1A deletion (DYT1) carriers manifesting dystonia. Parkinsonism Relat Disord 58:63–69
Ibrahim W, Murr N (2019) Lafora disease. StatPearls Publishing, Treasure Island
Ibrahim W, Sharma S (2018) Myoclonus, 2019/02/07 edition. StatPearls Publishing, Treasure Island
Intihar TA, Martinez EA, Gomez-Pastor R (2019) Mitochondrial dysfunction in Huntington’s disease; interplay between HSF1, p53 and Pgc-1alpha transcription factors. Front Cell Neurosci 13:103
Invernizzi F, Zorzi G, Legati A, Coppola G, D’Adamo P, Nardocci N, Garavaglia B, Ghezzi D (2018) Benign hereditary chorea and deletions outside NKX2-1: what’s the role of MBIP? Eur J Med Genet 61:581–584
Jahngir MU, Patel BC (2018) Meige syndrome, 2018/07/19 edition. StatPearls Publishing, Treasure Island
Jamal F (2019) Recent updates on essential tremor. Int J Neurol Neurother. 6:081. https://doi.org/10.23937/2378-3001/1410081
Jansen AC, Andermann E (2019) Progressive myoclonus epilepsy, Lafora type. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews® [Internet], 2019/02/21 edition. University of Washington, Seattle. http://www.ncbi.nlm.nih.gov/books/NBK1389. Accessed 3 June 2019
Jellinger KA (1998) Alzheimer-type lesions in Huntington’s disease. J Neural Transm 105:787–799
Jellinger KA (2011) Pallidal and thalamic atrophies. In: Gálvez-Jiménez N, Tuite PJ (eds) Uncommon causes of movement disorders. Cambridge University Press Cambridge, Cambridge, pp 94–107
Jellinger KA (2016a) Neuropathology of essential tremor. J Mult Scler 3:165. https://doi.org/10.4172/2376-0389.1000165
Jellinger KA (2016b) Neuropathology of movement disorders. In: Winn HR (ed) Youmans neurological surgery, vol 1, 7th edn. Elsevier-Saunders, Philadelphia, pp e840–e880
Jellinger KA (2017) Potential clinical utility of multiple system atrophy biomarkers. Expert Rev Neurother 17:1189–1208
Jellinger KA (2019) Is Braak staging valid for all types of Parkinson’s disease? J Neural Transm (Vienna) 126:423–431
Jeon I, Cicchetti F, Cisbani G, Lee S, Li E, Bae J, Lee N, Li L, Im W, Kim M, Kim HS, Oh SH, Kim TA, Ko JJ, Aube B, Oueslati A, Kim YJ, Song J (2016) Human-to-mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathol 132:577–592
Jinnah HA, Sun YV (2019) Dystonia genes and their biological pathways. Neurobiol Dis 129:159–168
Jinnah HA, Teller JK, Galpern WR (2015) Recent developments in dystonia. Curr Opin Neurol 28:400–405
Jinnah HA, Neychev V, Hess EJ (2017) The anatomical basis for dystonia: the motor network model. Tremor Other Hyperkinet Mov (N Y) 7:506
Johnson JA, Stacy M (2011) Unusual causes of tremor. In: Gálvez-Jiménez N, Tuite P (eds) Uncommon causes of movement disorders, 1st edn. Cambridge University Press, Cambridge, pp 153–163
Jones KS, Ramphul K (2019) Tourette syndrome and other tic disorders, 2018/05/16 edition. StatPearls Publishing, Treasure Island
Jung HH, Danek A, Walker RH (2011) Neuroacanthocytosis syndromes. Orphanet J Rare Dis 6:68
Jung HH, Danek A, Walker RH, Frey BM, Gassner C (2019) McLeod neuroacanthocytosis syndrome. In: GeneReviews® [Internet], 2019/05/23 edition. University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/NBK1354. Accessed 5 June 2019
Kaji R, Bhatia K, Graybiel AM (2018) Pathogenesis of dystonia: is it of cerebellar or basal ganglia origin? J Neurol Neurosurg Psychiatry 89:488–492
Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM (2005) Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA 102:13307–13312
Kaganovich D, Kopito R, Frydman J (2008) Misfolded proteins partition between two distinct quality control compartments. Nature 454:1088–1095
Kara E, Hardy J, Houlden H (2013) The pallidopyramidal syndromes: nosology, aetiology and pathogenesis. Curr Opin Neurol 26:381–394
Kawakami I, Kobayashi Z, Arai T, Yokota O, Nonaka T, Aoki N, Niizato K, Oshima K, Higashi S, Katsuse O, Hosokawa M, Hasegawa M, Akiyama H (2016) Chorea as a clinical feature of the basophilic inclusion body disease subtype of fused-in-sarcoma-associated frontotemporal lobar degeneration. Acta Neuropathol Commun 4:36
Keogh MJ, Morris CM, Chinnery PF (2013) Neuroferritinopathy. Int Rev Neurobiol 110:91–123
Kim HJ, Lee DH, Park JH (2008) Posttraumatic hemiballism with focal discrete hemorrhage in contralateral subthalamic nucleus. Parkinsonism Relat Disord 14:259–261
Kim EH, Thu DC, Tippett LJ, Oorschot DE, Hogg VM, Roxburgh R, Synek BJ, Waldvogel HJ, Faull RL (2014) Cortical interneuron loss and symptom heterogeneity in Huntington disease. Ann Neurol 75:717–727
Kim SY, Lee JS, Kim WJ, Kim H, Choi SA, Lim BC, Kim KJ, Chae JH (2018) Paroxysmal dyskinesia in children: from genes to the clinic. J Clin Neurol 14:492–497
Kleiner-Fisman G, Calingasan NY, Putt M, Chen J, Beal MF, Lang AE (2005) Alterations of striatal neurons in benign hereditary chorea. Mov Disord 20:1353–1357
Koeppen AH (2018) The neuropathology of spinocerebellar ataxia type 3/Machado-Joseph disease. Adv Exp Med Biol 1049:233–241
Kojovic M, Cordivari C, Bhatia K (2011) Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord 4:47–62
Koutsis G, Karadima G, Kartanou C, Kladi A, Panas M (2015) C9ORF72 hexanucleotide repeat expansions are a frequent cause of Huntington disease phenocopies in the Greek population. Neurobiol Aging 36(547):e513–546
Kovacs A, Kiss M, Pinter N, Szirmai I, Kamondi A (2019) Characteristics of tremor induced by lesions of the cerebellum. Cerebellum. https://doi.org/10.1007/s12311-12019-01027-12313
Kruer MC (2013) The neuropathology of neurodegeneration with brain iron accumulation. Int Rev Neurobiol 110:165–194
Kumar N, Rizek P, Jog M (2016) Neuroferritinopathy: pathophysiology, presentation, differential diagnoses and management. Tremor Other Hyperkinet Mov (N Y) 6:355
Kuo PH, Gan SR, Wang J, Lo RY, Figueroa KP, Tomishon D, Pulst SM, Perlman S, Wilmot G, Gomez CM, Schmahmann JD, Paulson H, Shakkottai VG, Ying SH, Zesiewicz T, Bushara K, Geschwind MD, Xia G, Subramony SH, Ashizawa T, Kuo SH (2017a) Dystonia and ataxia progression in spinocerebellar ataxias. Parkinsonism Relat Disord 45:75–80
Kuo SH, Lin CY, Wang J, Sims PA, Pan MK, Liou JY, Lee D, Tate WJ, Kelly GC, Louis ED, Faust PL (2017b) Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol 133:121–138
Kuo SH, Louis ED, Faust PL, Handforth A, Chang SY, Avlar B, Lang EJ, Pan MK, Miterko LN, Brown AM, Sillitoe RV, Anderson CJ, Pulst SM, Gallagher MJ, Lyman KA, Chetkovich DM, Clark LN, Tio M, Tan EK (2019) Current opinions and consensus for studying tremor in animal models. Cerebellum. https://doi.org/10.1007/s12311-12019-01037-12311
Kurian MA, Hayflick SJ (2013) Pantothenate kinase-associated neurodegeneration (PKAN) and PLA2G6-associated neurodegeneration (PLAN): review of two major neurodegeneration with brain iron accumulation (NBIA) phenotypes. Int Rev Neurobiol 110:49–71
Kurlan R, McDermott MP, Deeley C, Como PG, Brower C, Eapen S, Andresen EM, Miller B (2001) Prevalence of tics in schoolchildren and association with placement in special education. Neurology 57:1383–1388
Kuwata T, Okada Y, Yamamoto T, Sato D, Fujiwara K, Fukumura T, Ikeguchi M (2019) Structure, function, folding, and aggregation of a neuroferritinopathy-related ferritin variant. Biochemistry 58:2318–2325
Kuyper DJ, Parra V, Aerts S, Okun MS, Kluger BM (2011) Nonmotor manifestations of dystonia: a systematic review. Mov Disord 26:1206–1217
Lai SC, Jung SM, Grattan-Smith P, Sugo E, Lin YW, Chen RS, Chen CC, Wu-Chou YH, Lang AE, Lu CS (2010) Neuronal intranuclear inclusion disease: two cases of dopa-responsive juvenile parkinsonism with drug-induced dyskinesia. Mov Disord 25:1274–1279
Lai RY, Tomishon D, Figueroa KP, Pulst SM, Perlman S, Wilmot G, Gomez CM, Schmahmann JD, Paulson H, Shakkottai VG, Ying SH, Zesiewicz T, Bushara K, Geschwind M, Xia G, Subramony SH, Ashizawa T, Kuo SH (2019) Tremor in the degenerative cerebellum: towards the understanding of brain circuitry for tremor. Cerebellum. https://doi.org/10.1007/s12311-12019-01016-12316
Laurens B, Constantinescu R, Freeman R, Gerhard A, Jellinger K, Jeromin A, Krismer F, Mollenhauer B, Schlossmacher MG, Shaw LM, Verbeek MM, Wenning GK, Winge K, Zhang J, Meissner WG (2015) Fluid biomarkers in multiple system atrophy: a review of the MSA Biomarker Initiative. Neurobiol Dis 80:29–41
Lee D, Gan SR, Faust PL, Louis ED, Kuo SH (2018a) Climbing fiber-Purkinje cell synaptic pathology across essential tremor subtypes. Parkinsonism Relat Disord 51:24–29
Lee WW, Jeon B, Kim R (2018b) Expanding the spectrum of dopa-responsive dystonia (DRD) and proposal for new definition: DRD, DRD-plus, and DRD look-alike. J Korean Med Sci 33:e184
Lee PJ, Kerridge CA, Chatterjee D, Koeppen AH, Faust PL, Louis ED (2019) A quantitative study of empty baskets in essential tremor and other motor neurodegenerative diseases. J Neuropathol Exp Neurol 78:113–122
Lenka A, Louis ED (2018) Revisiting the clinical phenomenology of “cerebellar tremor”: beyond the intention tremor. Cerebellum. https://doi.org/10.1007/s12311-12018-10994-12316
Lenka A, Bhalsing KS, Panda R, Jhunjhunwala K, Naduthota RM, Saini J, Bharath RD, Yadav R, Pal PK (2017) Role of altered cerebello–thalamo–cortical network in the neurobiology of essential tremor. Neuroradiology 59:157–168
Levi S, Tiranti V (2019) Neurodegeneration with brain iron accumulation disorders: valuable models aimed at understanding the pathogenesis of iron deposition. Pharmaceuticals (Basel) 12:27
Levy A, Lang AE (2018) Ataxia-telangiectasia: a review of movement disorders, clinical features, and genotype correlations. Mov Disord 33:1238–1247
Lewczuk P, Riederer P, O’Bryant SE, Verbeek MM, Dubois B, Visser PJ, Jellinger KA, Engelborghs S, Ramirez A, Parnetti L, Jack CR Jr, Teunissen CE, Hampel H, Lleo A, Jessen F, Glodzik L, de Leon MJ, Fagan AM, Molinuevo JL, Jansen WJ, Winblad B, Shaw LM, Andreasson U, Otto M, Mollenhauer B, Wiltfang J, Turner MR, Zerr I, Handels R, Thompson AG, Johansson G, Ermann N, Trojanowski JQ, Karaca I, Wagner H, Oeckl P, van Waalwijk van Doorn L, Bjerke M, Kapogiannis D, Kuiperij HB, Farotti L, Li Y, Gordon BA, Epelbaum S, Vos SJB, Klijn CJM, Van Nostrand WE, Minguillon C, Schmitz M, Gallo C, Lopez Mato A, Thibaut F, Lista S, Alcolea D, Zetterberg H, Blennow K, Kornhuber J (2018) Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry 19:244–328
Lewis K, O’Day CS (2019) Dystonic Reactions, 2018/10/05 edition. StatPearls Publishing, Treasure Island
Li A, Paudel R, Johnson R, Courtney R, Lees AJ, Holton JL, Hardy J, Revesz T, Houlden H (2013) Pantothenate kinase-associated neurodegeneration is not a synucleinopathy. Neuropathol Appl Neurobiol 39:121–131
Liang CC, Tanabe LM, Jou S, Chi F, Dauer WT (2014) TorsinA hypofunction causes abnormal twisting movements and sensorimotor circuit neurodegeneration. J Clin Invest 124:3080–3092
Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH (2014) Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 137:3149–3159
Liu CS, Cheng WL, Kuo SJ, Li JY, Soong BW, Wei YH (2008) Depletion of mitochondrial DNA in leukocytes of patients with poly-Q diseases. J Neurol Sci 264:18–21
Liu AKL, Lim EJ, Ahmed I, Chang RC, Pearce RKB, Gentleman SM (2018a) Review: revisiting the human cholinergic nucleus of the diagonal band of Broca. Neuropathol Appl Neurobiol 44:647–662
Liu J, Heinsen H, Grinberg LT, Alho E, Amaro E Jr, Pasqualucci CA, Rub U, den Dunnen W, Arzberger T, Schmitz C, Kiessling M, Bader B, Danek A (2018b) Subcortical neurodegeneration in chorea: similarities and differences between chorea-acanthocytosis and Huntington’s disease. Parkinsonism Relat Disord 49:54–59
Liu J, Heinsen H, Grinberg LT, Alho E, Amaro E Jr, Pasqualucci CA, Rub U, Seidel K, den Dunnen W, Arzberger T, Schmitz C, Kiessling MC, Bader B, Danek A (2019) Pathoarchitectonics of the cerebral cortex in chorea-acanthocytosis and Huntington’s disease. Neuropathol Appl Neurobiol 45:230–243
Lohmann K, Klein C (2017) Update on the genetics of dystonia. Curr Neurol Neurosci Rep 17:26
Lorincz MT (2018) Wilson disease and related copper disorders. Handb Clin Neurol 147:279–292
Louis ED (2016) Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum 15:235–242
Louis ED (2018) Essential tremor and the cerebellum. Handb Clin Neurol 155:245–258
Louis ED (2019) The roles of age and aging in essential tremor: an epidemiological perspective. Neuroepidemiology 52:111–118
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N (2007) Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 130:3297–3307
Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP (2013) Quantification of cerebellar hemispheric Purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord 28:1854–1859
Louis ED, Kuo SH, Vonsattel JP, Faust PL (2014) Torpedo formation and Purkinje cell loss: modeling their relationship in cerebellar disease. Cerebellum 13:433–439
Louis ED, Ferrer M, Eliasen EH, Gaini S, Petersen MS (2019) Tremor in normal adults: a population-based study of 1158 adults in the Faroe Islands. J Neurol Sci 400:169–174
Luo R, Pan P, Xu Y, Chen L (2019) No reliable gray matter changes in essential tremor. Neurol Sci. https://doi.org/10.1007/s10072-10019-03933-10070
Ma K, Babij R, Cortes E, Vonsattel JP, Louis ED (2012) Cerebellar pathology of a dual clinical diagnosis: patients with essential tremor and dystonia. Tremor Other Hyperkinet Mov (N Y). https://doi.org/10.7916/D8JD4VJ5
Malone A, Manto M, Hass C (2014) Dissecting the links between cerebellum and dystonia. Cerebellum 13:666–668
Marchi G, Busti F, Lira Zidanes A, Castagna A, Girelli D (2019) Aceruloplasminemia: a severe neurodegenerative disorder deserving an early diagnosis. Front Neurosci 13:325
Marras C, Lang A, van de Warrenburg BP, Sue CM, Tabrizi SJ, Bertram L, Mercimek-Mahmutoglu S, Ebrahimi-Fakhari D, Warner TT, Durr A, Assmann B, Lohmann K, Kostic V, Klein C (2017) Nomenclature of genetic movement disorders: recommendations of the International Parkinson and Movement Disorder Society task force. Mov Disord 32:724–725
Martino D, Hedderly T (2019) Tics and stereotypies: a comparative clinical review. Parkinsonism Relat Disord 59:117–124
Martino D, Ganos C, Pringsheim TM (2017) Tourette syndrome and chronic tic disorders: the clinical spectrum beyond tics. Int Rev Neurobiol 134:1461–1490
Masnata M, Sciacca G, Maxan A, Bousset L, Denis HL, Lauruol F, David L, Saint-Pierre M, Kordower JH, Melki R, Alpaugh M, Cicchetti F (2019) Demonstration of prion-like properties of mutant huntingtin fibrils in both in vitro and in vivo paradigms. Acta Neuropathol 137:981–1001
Matos CA, de Almeida LP, Nobrega C (2019) Machado-Joseph disease/spinocerebellar ataxia type 3: lessons from disease pathogenesis and clues into therapy. J Neurochem 148:8–28
Matsumoto A, Suzuki H, Fukatsu R, Shimizu H, Suzuki Y, Hisanaga K (2016) An autopsy case of frontotemporal lobar degeneration with the appearance of fused in sarcoma inclusions (basophilic inclusion body disease) clinically presenting corticobasal syndrome. Neuropathology 36:77–87
Mehrabi NF, Singh-Bains MK, Waldvogel HJ, Faull RLM (2016) Cortico-basal ganglia interactions in Huntington’s disease. Ann Neurodegen Disord 1:1007
Mehta S, Goyal MK, Kilbane C, Kumar R, Lal V (2018) An unusual and intriguing presentation of Sydenham’s chorea. Tremor Other Hyperkinet Mov (N Y) 8:593
Merical B, Sanchez-Manso JC (2019) Chorea, 2017/06/15 edition. StatPearls Publishing, Treasure Island
Meringolo M, Tassone A, Imbriani P, Ponterio G, Pisani A (2018) Dystonia: are animal models relevant in therapeutics? Rev Neurol (Paris) 174:608–614
Mi Y, Gao X, Xu H, Cui Y, Zhang Y, Gou X (2019) The emerging roles of ferroptosis in Huntington’s disease. Neuromol Med 21:110–119
Mills K, Mari Z (2015) An update and review of the treatment of myoclonus. Curr Neurol Neurosci Rep 15:512
Mostile G, Barone R, Nicoletti A, Rizzo R, Martinelli D, Sturiale L, Fiumara A, Jankovic J, Zappia M (2019) Hyperkinetic movement disorders in congenital disorders of glycosylation. Eur J Neurol. https://doi.org/10.1111/ene.14007
Moutton S, Fergelot P, Trocello JM, Plante-Bordeneuve V, Houcinat N, Wenisch E, Larue V, Brugieres P, Clot F, Lacombe D, Arveiler B, Goizet C (2014) A novel FTL mutation responsible for neuroferritinopathy with asymmetric clinical features and brain anomalies. Parkinsonism Relat Disord 20:935–937
Muller-Vahl KR, Sambrani T, Jakubovski E (2019) Tic disorders revisited: introduction of the term “tic spectrum disorders”. Eur Child Adolesc Psychiatry. https://doi.org/10.1007/s00787-00018-01272-00787
Munoz DG, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, Kuroda S, Mackenzie IR (2009) FUS pathology in basophilic inclusion body disease. Acta Neuropathol 118:617–627
Munoz-Braceras S, Tornero-Ecija AR, Vincent O, Escalante R (2019) VPS13A is closely associated with mitochondria and is required for efficient lysosomal degradation. Dis Model Mech. https://doi.org/10.1242/dmm.036681
Nana AL, Kim EH, Thu DC, Oorschot DE, Tippett LJ, Hogg VM, Synek BJ, Roxburgh R, Waldvogel HJ, Faull RL (2014) Widespread heterogeneous neuronal loss across the cerebral cortex in Huntington’s disease. J Huntingt Dis 3:45–64
Nethisinghe S, Lim WN, Ging H, Zeitlberger A, Abeti R, Pemble S, Sweeney MG, Labrum R, Cervera C, Houlden H, Rosser E, Limousin P, Kennedy A, Lunn MP, Bhatia KP, Wood NW, Hardy J, Polke JM, Veneziano L, Brusco A, Davis MB, Giunti P (2018) Complexity of the genetics and clinical presentation of spinocerebellar ataxia 17. Front Cell Neurosci 12:429
Newby R, Alty J, Kempster P (2016) Functional dystonia and the borderland between neurology and psychiatry: new concepts. Mov Disord 31:1777–1784
Nicoletti V, Cecchi P, Frosini D, Pesaresi I, Fabbri S, Diciotti S, Bonuccelli U, Cosottini M, Ceravolo R (2015) Morphometric and functional MRI changes in essential tremor with and without resting tremor. J Neurol 262:719–728
Nishida K, Garringer HJ, Futamura N, Funakawa I, Jinnai K, Vidal R, Takao M (2014) A novel ferritin light chain mutation in neuroferritinopathy with an atypical presentation. J Neurol Sci 342:173–177
Nithianantharajah J, Hannan AJ (2013) Dysregulation of synaptic proteins, dendritic spine abnormalities and pathological plasticity of synapses as experience-dependent mediators of cognitive and psychiatric symptoms in Huntington’s disease. Neuroscience 251:66–74
Nitschke F, Ahonen SJ, Nitschke S, Mitra S, Minassian BA (2018) Lafora disease—from pathogenesis to treatment strategies. Nat Rev Neurol 14:606–617
Nomura Y (2017) Tourette syndrome: clinical features and pathophysiology. Brain Nerve 69:1373–1385
Nopoulos PC (2016) Huntington disease: a single-gene degenerative disorder of the striatum. Dialogues Clin Neurosci 18:91–98
Oblak AL, Hagen MC, Sweadner KJ, Haq I, Whitlow CT, Maldjian JA, Epperson F, Cook JF, Stacy M, Murrell JR, Ozelius LJ, Brashear A, Ghetti B (2014) Rapid-onset dystonia-Parkinsonism associated with the I758S mutation of the ATP1A3 gene: a neuropathologic and neuroanatomical study of four siblings. Acta Neuropathol 128:81–98
Oh SY, Kim JS, Dieterich M (2019) Update on opsoclonus-myoclonus syndrome in adults. J Neurol 266:1541–1548
Pan JJ, Lee M, Honig LS, Vonsattel JP, Faust PL, Louis ED (2014) Alzheimer’s-related changes in non-demented essential tremor patients vs. controls: links between tau and tremor? Parkinsonism Relat Disord 20:655–658
Pan MK, Ni CL, Wu YC, Li YS, Kuo SH (2018) Animal models of tremor: relevance to human tremor disorders. Tremor Other Hyperkinet Mov (N Y) 8:587
Pana A, Saggu BM (2019) Dystonia, 2017/08/29 edition. StatPearls Publishing, Treasure Island
Papoutsi M, Labuschagne I, Tabrizi SJ, Stout JC (2014) The cognitive burden in Huntington’s disease: pathology, phenotype, and mechanisms of compensation. Mov Disord 29:673–683
Paschou P (2013) The genetic basis of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 37:1026–1039
Paudel R, Li A, Hardy J, Bhatia KP, Houlden H, Holton J (2016) DYT6 Ddstonia: a neuropathological study. Neurodegener Dis 16:273–278
Paul BD, Snyder SH (2019) Impaired redox signaling in Huntington’s disease: therapeutic implications. Front Mol Neurosci 12:68
Peikert K, Danek A, Hermann A (2018) Current state of knowledge in chorea-acanthocytosis as core neuroacanthocytosis syndrome. Eur J Med Genet 61:699–705
Pietracupa S, Bruno E, Cavanna AE, Falla M, Zappia M, Colosimo C (2015) Scales for hyperkinetic disorders: a systematic review. J Neurol Sci 358:9–21
Podvin S, Reardon HT, Yin K, Mosier C, Hook V (2019) Multiple clinical features of Huntington’s disease correlate with mutant HTT gene CAG repeat lengths and neurodegeneration. J Neurol 266:551–564
Pogorelov V, Xu M, Smith HR, Buchanan GF, Pittenger C (2015) Corticostriatal interactions in the generation of tic-like behaviors after local striatal disinhibition. Exp Neurol 265:122–128
Potter KA, Kern MJ, Fullbright G, Bielawski J, Scherer SS, Yum SW, Li JJ, Cheng H, Han X, Venkata JK, Khan PA, Rohrer B, Hama H (2011) Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia 59:1009–1021
Poudel GR, Harding IH, Egan GF, Georgiou-Karistianis N (2019) Network spread determines severity of degeneration and disconnection in Huntington's disease. Hum Brain Mapp. https://doi.org/10.1002/hbm.24695
Poujois A, Mikol J, Woimant F (2017) Wilson disease: brain pathology. Handb Clin Neurol 142:77–89
Prasad S, Shah A, Bhalsing KS, Ingalhalikar M, Saini J, Pal PK (2019a) Clinical correlates of abnormal subcortical volumes in essential tremor. J Neural Transm (Vienna) 126:569–576
Prasad S, Shah A, Bhalsing KS, Kumar KJ, Saini J, Ingalhalikar M, Pal PK (2019b) Abnormal hippocampal subfields are associated with cognitive impairment in essential tremor. J Neural Transm (Vienna) 126:597–606
Prasad S, Pandey U, Saini J, Ingalhalikar M, Pal PK (2019c) Atrophy of cerebellar peduncles in essential tremor: a machine learning-based volumetric analysis. Eur Radiol. https://doi.org/10.1007/s00330-00019-06269-00337
Prell T, Peschel T, Kohler B, Bokemeyer MH, Dengler R, Gunther A, Grosskreutz J (2013) Structural brain abnormalities in cervical dystonia. BMC Neurosci 14:123
Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, Ledoux MS, Jinnah HA (2013) Neuropathology of cervical dystonia. Exp Neurol 241:95–104
Quarrell OW, Nance MA, Nopoulos P, Paulsen JS, Smith JA, Squitieri F (2013) Managing juvenile Huntington’s disease. Neurodegener Dis Manag 3:267–276
Rajan S, Kaas B, Moukheiber E (2019) Movement disorders emergencies. Semin Neurol 39:125–136
Rajput AH, Adler CH, Shill HA, Rajput A (2012) Essential tremor is not a neurodegenerative disease. Neurodegener Dis Manag 2:259–268
Rajput AH, Rajput EF, Bocking SM, Auer RN, Rajput A (2019) Parkinsonism in essential tremor cases: A clinicopathological study. Mov Disord. https://doi.org/10.1002/mds.27729
Ramachandran TS (2017) Chorea gravidarum. In: Benbadis SR, Talavera F, Galvez-Jimenez N (eds) Medscape [Internet]. WebMD LLC, New York, NY. https://www.emedicine.medscape.com/article/1149725-overview. Accessed 5 June 2019
Ramos A, Planchat M, Vieira Melo AR, Raposo M, Shamim U, Suroliya V, Srivastava AK, Faruq M, Morino H, Ohsawa R, Kawakami H, Bannach Jardim L, Saraiva-Pereira ML, Vasconcelos J, Santos C, Lima M (2019) Mitochondrial DNA haplogroups and age at onset of Machado–Joseph disease/spinocerebellar ataxia type 3: a study in patients from multiple populations. Eur J Neurol 26:506–512
Rangarh P, Kohli N (2018) Neuroimaging findings in Menkes disease: a rare neurodegenerative disorder. BMJ Case Rep 2018:bcr-2017-223858
Raposo M, Ramos A, Santos C, Kazachkova N, Teixeira B, Bettencourt C, Lima M (2019) Accumulation of mitochondrial DNA common deletion since the preataxic stage of Machado–Joseph disease. Mol Neurobiol 56:119–124
Rawlins MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJ, Smeeth L (2016) The prevalence of Huntington’s disease. Neuroepidemiology 46:144–153
Razmeh S, Habibi AH, Orooji M, Alizadeh E, Moradiankokhdan K, Razmeh B (2018) Pantothenate kinase-associated neurodegeneration: clinical aspects, diagnosis and treatments. Neurol Int 10:7516
Reich SG (2019) Essential tremor. Med Clin N Am 103:351–356
Reimao S, Pita Lobo P, Neutel D, Guedes LC, Coelho M, Rosa MM, Azevedo P, Ferreira J, Abreu D, Goncalves N, Nunes RG, Campos J, Ferreira JJ (2015) Substantia nigra neuromelanin-MR imaging differentiates essential tremor from Parkinson’s disease. Mov Disord 30:953–959
Riku Y, Ikeuchi T, Yoshino H, Mimuro M, Mano K, Goto Y, Hattori N, Sobue G, Yoshida M (2013) Extensive aggregation of alpha-synuclein and tau in juvenile-onset neuroaxonal dystrophy: an autopsied individual with a novel mutation in the PLA2G6 gene-splicing site. Acta Neuropathol Commun 1:12
Robottom BJ, Weiner WJ (2011) Chorea gravidarum. Handb Clin Neurol 100:231–235
Rohani M, Shahidi G, Alavi A, Lang AE, Yousefi N, Razme S, Fasano A (2017) Tremor-dominant pantothenate kinase-associated neurodegeneration. Mov Disord Clin Pract 4:772–774
Rose SJ, Yu XY, Heinzer AK, Harrast P, Fan X, Raike RS, Thompson VB, Pare JF, Weinshenker D, Smith Y, Jinnah HA, Hess EJ (2015) A new knock-in mouse model of l-DOPA-responsive dystonia. Brain 138:2987–3002
Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ (2014) Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 10:204–216
Roulis E, Hyland C, Flower R, Gassner C, Jung HH, Frey BM (2018) Molecular basis and clinical overview of McLeod syndrome compared with other neuroacanthocytosis syndromes: a review. JAMA Neurol 75:1554–1562
Roy A, Coombes SA, Chung JW, Archer DB, Okun MS, Hess CW, Wagle Shukla A, Vaillancourt DE (2019) Cortical dynamics within and between parietal and motor cortex in essential tremor. Mov Disord 34:95–104
Roze E, Lang AE, Vidailhet M (2018) Myoclonus-dystonia: classification, phenomenology, pathogenesis, and treatment. Curr Opin Neurol 31:484–490
Rub U, Hoche F, Brunt ER, Heinsen H, Seidel K, Del Turco D, Paulson HL, Bohl J, von Gall C, Vonsattel JP, Korf HW, den Dunnen WF (2013) Degeneration of the cerebellum in Huntington’s disease (HD): possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol 23:165–177
Rub U, Hentschel M, Stratmann K, Brunt E, Heinsen H, Seidel K, Bouzrou M, Auburger G, Paulson H, Vonsattel JP, Lange H, Korf HW, den Dunnen W (2014) Huntington’s disease (HD): degeneration of select nuclei, widespread occurrence of neuronal nuclear and axonal inclusions in the brainstem. Brain Pathol 24:247–260
Rub U, Vonsattel JP, Heinsen H, Korf HW (2015) The neuropathology of Huntington s disease: classical findings, recent developments and correlation to functional neuroanatomy. Adv Anat Embryol Cell Biol 217:1–146
Rub U, Seidel K, Heinsen H, Vonsattel JP, den Dunnen WF, Korf HW (2016) Huntington’s disease (HD): the neuropathology of a multisystem neurodegenerative disorder of the human brain. Brain Pathol 26:726–740
Rudakou U, Ouled Amar Bencheikh B, Ruskey JA, Krohn L, Laurent SB, Spiegelman D, Liong C, Fahn S, Waters C, Monchi O, Fon EA, Dauvilliers Y, Alcalay RN, Dupre N, Gan-Or Z (2019) Common and rare GCH1 variants are associated with Parkinson’s disease. Neurobiol Aging 73:231 e231–231 e236
Sadnicka A, Galea JM, Chen JC, Warner TT, Bhatia KP, Rothwell JC, Edwards MJ (2018) Delineating cerebellar mechanisms in DYT11 myoclonus-dystonia. Mov Disord 33:1956–1961
Saifee TA (2019) Tremor. Br Med Bull. https://doi.org/10.1093/bmb/ldz1017
Salgado P, Taipa R, Domingos J, Dias D, Pires MM, Magalhaes M (2017) Vascular pathology causing late onset generalized chorea: a clinico-pathological case report. Mov Disord Clin Pract 4:819–823
Schneider SA, Bird T (2016) Huntington’s disease, Huntington’s disease look-alikes, and benign hereditary chorea: what’s new? Mov Disord Clin Pract 3:342–354
Schneider SA, Hardy J, Bhatia KP (2012) Syndromes of neurodegeneration with brain iron accumulation (NBIA): an update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Mov Disord 27:42–53
Sedov A, Usova S, Semenova U, Gamaleya A, Tomskiy A, Crawford JD, Corneil B, Jinnah HA, Shaikh AG (2019) The role of pallidum in the neural integrator model of cervical dystonia. Neurobiol Dis 125:45–54
Shacham T, Sharma N, Lederkremer GZ (2019) Protein misfolding and er stress in Huntington’s disease. Front Mol Biosci 6:20
Shakkottai VG, Batla A, Bhatia K, Dauer WT, Dresel C, Niethammer M, Eidelberg D, Raike RS, Smith Y, Jinnah HA, Hess EJ, Meunier S, Hallett M, Fremont R, Khodakhah K, LeDoux MS, Popa T, Gallea C, Lehericy S, Bostan AC, Strick PL (2017) Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum 16:577–594
Sharma N (2019) Neuropathology of dystonia. Tremor Other Hyperkinet Mov (N Y) 9:569
Shetty AS, Bhatia KP, Lang AE (2019) Dystonia and Parkinson’s disease: what is the relationship? Neurobiol Dis. https://doi.org/10.1016/j.nbd.2019.1005.1001
Shibasaki H (2006) Myoclonus and startle syndromes. In: Jankovic JJ, Tolosa E (eds) Parkinson’s disease and movement disorders, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 376–386
Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, Beach TG (2008) Pathologic findings in prospectively ascertained essential tremor subjects. Neurology 70:1452–1455
Siebert M, Donis KC, Socal M, Rieder CR, Emmel VE, Vairo F, Michelin-Tirelli K, Franca M Jr, D’Abreu AC, Bettencourt C, Lima M, Lopes Cendes I, Saraiva-Pereira ML, Jardim LB (2012) Glucocerebrosidase gene variants in parkinsonian patients with Machado Joseph/spinocerebellar ataxia 3. Parkinsonism Relat Disord 18:185–190
Simonyan K (2019) Recent advances in understanding the role of the basal ganglia [version 1; peer review: 2 approved]. F1000Res 8(F1000 Faculty Rev):122. https://doi.org/10.12688/f1000research.16524.1
Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL (2008) Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain 131:447–459
Simonyan K, Ludlow CL, Vortmeyer AO (2010) Brainstem pathology in spasmodic dysphonia. Laryngoscope 120:121–124
Singer HS, Mink JW, Gilbert DL, Jankovic J (2010) Movement disorders in childhood. Saunders Elsevier, Philadelphia
Singh-Bains MK, Tippett LJ, Hogg VM, Synek BJ, Roxburgh RH, Waldvogel HJ, Faull RL (2016) Globus pallidus degeneration and clinicopathological features of Huntington disease. Ann Neurol 80:185–201
Singh-Bains MK, Mehrabi NF, Sehji T, Austria MDR, Tan AYS, Tippett LJ, Dragunow M, Waldvogel HJ, Faull RLM (2019) Cerebellar degeneration correlates with motor symptoms in Huntington disease. Ann Neurol 85:396–405
Siokas V, Aloizou AM, Tsouris Z, Michalopoulou A, Mentis AA, Dardiotis E (2019) Risk factor genes in patients with dystonia: a comprehensive review. Tremor Other Hyperkinet Mov (N Y) 8:559
Sittler A, Muriel MP, Marinello M, Brice A, den Dunnen W, Alves S (2018) Deregulation of autophagy in postmortem brains of Machado–Joseph disease patients. Neuropathology 38:113–124
Smit M, Bartels AL, van Faassen M, Kuiper A, Niezen-Koning KE, Kema IP, Dierckx RA, de Koning TJ, Tijssen MA (2016) Serotonergic perturbations in dystonia disorders—a systematic review. Neurosci Biobehav Rev 65:264–275
Smith-Dijak AI, Sepers MD, Raymond LA (2019) Alterations in synaptic function and plasticity in Huntington disease. J Neurochem. https://doi.org/10.1111/jnc.14723
Soares TR, Reis SD, Pinho BR, Duchen MR, Oliveira JMA (2019) Targeting the proteostasis network in Huntington’s disease. Ageing Res Rev 49:92–103
Sone J, Sobue G (2017) Neuronal intranuclear inclusion disease. Brain Nerve 69:5–16
Sone J, Tanaka F, Koike H, Inukai A, Katsuno M, Yoshida M, Watanabe H, Sobue G (2011) Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology 76:1372–1376
Sone D, Sato N, Yokoyama K, Sumida K, Kanai M, Imabayashi E, Saito Y, Matsuda H (2016a) Striatal glucose hypometabolism in preadolescent-onset dentatorubral–pallidoluysian atrophy. J Neurol Sci 360:121–124
Sone J, Mori K, Inagaki T, Katsumata R, Takagi S, Yokoi S, Araki K, Kato T, Nakamura T, Koike H, Takashima H, Hashiguchi A, Kohno Y, Kurashige T, Kuriyama M, Takiyama Y, Tsuchiya M, Kitagawa N, Kawamoto M, Yoshimura H, Suto Y, Nakayasu H, Uehara N, Sugiyama H, Takahashi M, Kokubun N, Konno T, Katsuno M, Tanaka F, Iwasaki Y, Yoshida M, Sobue G (2016b) Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 139:3170–3186
Stamelou M, Charlesworth G, Cordivari C, Schneider SA, Kagi G, Sheerin UM, Rubio-Agusti I, Batla A, Houlden H, Wood NW, Bhatia KP (2014) The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord 29:928–934
St-Amour I, Turgeon A, Goupil C, Planel E, Hebert SS (2018) Co-occurrence of mixed proteinopathies in late-stage Huntington’s disease. Acta Neuropathol 135:249–265
Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T (2012) The prevalence of primary dystonia: a systematic review and meta-analysis. Mov Disord 27:1789–1796
Sugiyama A, Sato N, Nakata Y, Kimura Y, Enokizono M, Maekawa T, Kondo M, Takahashi Y, Kuwabara S, Matsuda H (2018) Clinical and magnetic resonance imaging features of elderly onset dentatorubral–pallidoluysian atrophy. J Neurol 265:322–329
Sullivan MA, Nitschke S, Steup M, Minassian BA, Nitschke F (2017) Pathogenesis of Lafora disease: transition of soluble glycogen to insoluble polyglucosan. Int J Mol Sci 18:1743
Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, Driver-Dunckley E, Beach TG (2014) Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord 29:496–500
Tada M, Coon EA, Osmand AP, Kirby PA, Martin W, Wieler M, Shiga A, Shirasaki H, Makifuchi T, Yamada M, Kakita A, Nishizawa M, Takahashi H, Paulson HL (2012) Coexistence of Huntington’s disease and amyotrophic lateral sclerosis: a clinicopathologic study. Acta Neuropathol 124:749–760
Takahashi H, Yamada M, Tsuji S (2011) Dentatorubral–pallidoluysian atrophy. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 299–306
Takahashi-Fujigasaki J, Nakano Y, Uchino A, Murayama S (2016) Adult-onset neuronal intranuclear hyaline inclusion disease is not rare in older adults. Geriatr Gerontol Int 16(Suppl 1):51–56
Tan Z, Dai W, van Erp TG, Overman J, Demuro A, Digman MA, Hatami A, Albay R, Sontag EM, Potkin KT, Ling S, Macciardi F, Bunney WE, Long JD, Paulsen JS, Ringman JM, Parker I, Glabe C, Thompson LM, Chiu W, Potkin SG (2015) Huntington’s disease cerebrospinal fluid seeds aggregation of mutant huntingtin. Mol Psychiatry 20:1286–1293
Tarakad A, Jankovic J (2019) Essential tremor and Parkinson’s disease: exploring the relationship. Tremor Other Hyperkinet Mov (N Y) 8:589
Tereshchenko A, Magnotta V, Epping E, Mathews K, Espe-Pfeifer P, Martin E, Dawson J, Duan W, Nopoulos P (2019) Brain structure in juvenile-onset Huntington disease. Neurology 92:e1939–e1947
Testa CM, Jankovic J (2019) Huntington disease: a quarter century of progress since the gene discovery. J Neurol Sci 396:52–68
Tewari A, Fremont R, Khodakhah K (2017) It’s not just the basal ganglia: cerebellum as a target for dystonia therapeutics. Mov Disord 32:1537–1545
Tippett LJ, Waldvogel HJ, Snell RG, Vonsattel JP, Young AB, Faull RLM (2017) The complexity of clinical Huntington’s disease: developments in molecular genetics, neuropathology and neuroimaging biomarkers. Adv Neurobiol 15:129–161
Tofaris GK, Revesz T, Jacques TS, Papacostas S, Chataway J (2007) Adult-onset neurodegeneration with brain iron accumulation and cortical alpha-synuclein and tau pathology: a distinct clinicopathological entity. Arch Neurol 64:280–282
Tonduti D, Chiapparini L, Moroni I, Ardissone A, Zorzi G, Zibordi F, Raspante S, Panteghini C, Garavaglia B, Nardocci N (2016) Neurological disorders associated with striatal lesions: classification and diagnostic approach. Curr Neurol Neurosci Rep 16:54
Truong DD, Frei K (2018) Setting the record straight: the nosology of tardive syndromes. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.1011.1025 (online Nov 28)
Tsuji S (2012) Dentatorubral–pallidoluysian atrophy. Handb Clin Neurol 103:587–594
Turnbull J, Tiberia E, Striano P, Genton P, Carpenter S, Ackerley CA, Minassian BA (2016) Lafora disease. Epileptic Disord 18:38–62
Uchino A, Ogino M, Takahashi-Fujigasaki J, Oonuma S, Kanazawa N, Kajita S, Ichinoe M, Hasegawa M, Nishiyama K, Murayama S (2018) Pathological and immunoblot analysis of phosphorylated TDP-43 in sporadic amyotrophic lateral sclerosis with pallido-nigro-luysian degeneration. Neuropathology 38:171–178
Uehara K, Furuya S, Numazawa H, Kita K, Sakamoto T, Hanakawa T (2019) Distinct roles of brain activity and somatotopic representation in pathophysiology of focal dystonia. Hum Brain Mapp 40:1738–1749
Ure RJ, Dhanju S, Lang AE, Fasano A (2016) Unusual tremor syndromes: know in order to recognise. J Neurol Neurosurg Psychiatry 87:1191–1203
Vairo FPE, Chwal BC, Perini S, Ferreira MAP, de Freitas Lopes AC, Saute JAM (2019) A systematic review and evidence-based guideline for diagnosis and treatment of Menkes disease. Mol Genet Metab 126:6–13
Vanni V, Puglisi F, Bonsi P, Ponterio G, Maltese M, Pisani A, Mandolesi G (2015) Cerebellar synaptogenesis is compromised in mouse models of DYT1 dystonia. Exp Neurol 271:457–467
Velayos Baeza A, Dobson-Stone C, Rampoldi L, Bader B, Walker RH, Danek A, Monaco AP (2019) Chorea-acanthocytosis. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews® [Internet], 2019/04/18 edition. University of Washington, Seattle. http://www.ncbi.nlm.nih.gov/books/NBK1387. Accessed 5 June 2019
Verhalen B, Arnold S, Minassian BA (2018) Lafora disease: a review of molecular mechanisms and pathology. Neuropediatrics 49:357–362
Versace V, Campostrini S, Sebastianelli L, Soda M, Saltuari L, Lun S, Nardone R, Kofler M (2019) Adult-onset Gilles de la Tourette syndrome: psychogenic or organic? The challenge of abnormal neurophysiological findings. Front Neurol 10:461
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577
Vonsattel JP, Keller C, Cortes Ramirez EP (2011) Huntington’s disease—neuropathology. Handb Clin Neurol 100:83–100
Vroegindeweij LHP, Boon AJW, Wilson JHP, Langendonk JG (2019) Aceruloplasminemia: neurodegeneration with brain iron accumulation (NBIA) associated with psychosis. J Inherit Metab Dis 42:195–196
Waldvogel HJ, Kima EH, Thuc DCV, Tippett LJ, Faull RLM (2012) New perspectives on the neuropathology in Huntington’s disease in the human brain and its relation to symptom variation. J Huntingt Dis 1:143–153
Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL (2015) The neuropathology of Huntington’s disease. In: Nguyen HHP, Cenci MA (eds) Behavioral neurobiology of Huntington’s disease and Parkinson’s disease. Springer, Berlin, pp 33–80
Wang L, Chen Y, Hu B, Hu X (2016) Late-onset primary dystonia in Zhejiang province of China: a service-based epidemiological study. Neurol Sci 37:111–116
Wardle M, Morris HR, Robertson NP (2009) Clinical and genetic characteristics of non-Asian dentatorubral–pallidoluysian atrophy: a systematic review. Mov Disord 24:1636–1640
Weaver J, Sarva H, Barone D, Bobker S, Bushara K, Hiller A, Ishii M, Jankovic J, Lakhani S, Niotis K, Scharre DW, Tuite P, Stutz A, Westhoff CM, Walker RH (2019) McLeod syndrome: five new pedigrees with novel mutations. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2019.1004.1022
Westenberger A, Reyes CJ, Saranza G, Dobricic V, Hanssen H, Domingo A, Laabs BH, Schaake S, Pozojevic J, Rakovic A, Grutz K, Begemann K, Walter U, Dressler D, Bauer P, Rolfs A, Munchau A, Kaiser FJ, Ozelius LJ, Jamora RD, Rosales RL, Diesta CCE, Lohmann K, Konig IR, Bruggemann N, Klein C (2019) A hexanucleotide repeat modifies expressivity of X-linked dystonia parkinsonism. Ann Neurol 85:812–822
Wiatr K, Szlachcic WJ, Trzeciak M, Figlerowicz M, Figiel M (2018) Huntington disease as a neurodevelopmental disorder and early signs of the disease in stem cells. Mol Neurobiol 55:3351–3371
Wichmann T, Bergman H, DeLong MR (2018) Basal ganglia, movement disorders and deep brain stimulation: advances made through non-human primate research. J Neural Transm (Vienna) 125:419–430
Wijemanne S, Jankovic J (2015) Dopa-responsive dystonia—clinical and genetic heterogeneity. Nat Rev Neurol 11:414–424
Williams L, McGovern E, Kimmich O, Molloy A, Beiser I, Butler JS, Molloy F, Logan P, Healy DG, Lynch T, Walsh R, Cassidy L, Moriarty P, Moore H, McSwiney T, Walsh C, O’Riordan S, Hutchinson M (2017) Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland. Eur J Neurol 24:73–81
Wiltshire KM, Dunham C, Reid S, Auer RN, Suchowersky O (2010) Neuronal intranuclear inclusion disease presenting as juvenile parkinsonism. Can J Neurol Sci 37:213–218
Winder JY, Achterberg WP, Gardiner SL, Roos RAC (2019) Longitudinal assessment of the Unified Huntington’s Disease Rating Scale (UHDRS) and UHDRS-For Advanced Patients (UHDRS-FAP) in patients with late stage Huntington’s disease. Eur J Neurol 26:780–785
Woltjer RL, Reese LC, Richardson BE, Tran H, Green S, Pham T, Chalupsky M, Gabriel I, Light T, Sanford L, Jeong SY, Hamada J, Schwanemann LK, Rogers C, Gregory A, Hogarth P, Hayflick SJ (2015) Pallidal neuronal apolipoprotein E in pantothenate kinase-associated neurodegeneration recapitulates ischemic injury to the globus pallidus. Mol Genet Metab 116:289–297
Wong JC, Armstrong MJ, Lang AE, Hazrati LN (2013) Clinicopathological review of pallidonigroluysian atrophy. Mov Disord 28:274–281
Worbe Y, Sgambato-Faure V, Epinat J, Chaigneau M, Tande D, Francois C, Feger J, Tremblay L (2013) Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex 49:1126–1140
Wright GEB, Collins JA, Kay C, McDonald C, Dolzhenko E, Xia Q, Becanovic K, Drogemoller BI, Semaka A, Nguyen CM, Trost B, Richards F, Bijlsma EK, Squitieri F, Ross CJD, Scherer SW, Eberle MA, Yuen RKC, Hayden MR (2019) Length of uninterrupted CAG, independent of polyglutamine size, results in increased somatic instability, hastening onset of Huntington disease. Am J Hum Genet 104:1116–1126
Xu Q, Huang S, Song M, Wang CE, Yan S, Liu X, Gaertig MA, Yu SP, Li H, Li S, Li XJ (2013) Synaptic mutant huntingtin inhibits synapsin-1 phosphorylation and causes neurological symptoms. J Cell Biol 202:1123–1138
Yadav N, Raja P, Shetty SS, Jitender S, Prasad C, Kamble NL, Mahadevan A, MN, (2019) Neuronal intranuclear inclusion disease: a rare etiology for rapidly progressive dementia. Alzheimer Dis Assoc Disord. https://doi.org/10.1097/WAD.0000000000000312
Yamada M (2010) Dentatorubral–pallidoluysian atrophy (DRPLA): the 50th anniversary of Japanese Society of Neuropathology. Neuropathology 30:453–457
Yamaguchi N, Mano T, Ohtomo R, Ishiura H, Almansour MA, Mori H, Kanda J, Shirota Y, Taira K, Morikawa T, Ikemura M, Yanagi Y, Murayama S, Shimizu J, Sakurai Y, Tsuji S, Iwata A (2018) An autopsy case of familial neuronal intranuclear inclusion disease with dementia and neuropathy. Intern Med 57:3459–3462
Yano H, Baranov SV, Baranova OV, Kim J, Pan Y, Yablonska S, Carlisle DL, Ferrante RJ, Kim AH, Friedlander RM (2014) Inhibition of mitochondrial protein import by mutant huntingtin. Nat Neurosci 17:822–831
Yokoi F, Cheetham CC, Campbell SL, Sweatt JD, Li Y (2013) Pre-synaptic release deficits in a DYT1 dystonia mouse model. PLoS One 8:e72491
Yokota O, Tsuchiya K, Terada S, Ishizu H, Uchikado H, Ikeda M, Oyanagi K, Nakano I, Murayama S, Kuroda S, Akiyama H (2008) Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 115:561–575
Yoshida Y, Nunomura J, Shimohata T, Nanjo H, Miyata H (2012) Benign hereditary chorea 2: pathological findings in an autopsy case. Neuropathology 32:557–565
Yoshimoto T, Takamatsu K, Kurashige T, Sone J, Sobue G, Kuriyama M (2017) Adult-onset neuronal intranuclear inclusion disease in two female siblings. Brain Nerve 69:267–274
Yoshino H, Nishioka K, Li Y, Oji Y, Oyama G, Hatano T, Machida Y, Shimo Y, Hayashida A, Ikeda A, Mogushi K, Shibagaki Y, Hosaka A, Iwanaga H, Fujitake J, Ohi T, Miyazaki D, Sekijima Y, Oki M, Kusaka H, Fujimoto KI, Ugawa Y, Funayama M, Hattori N (2018) GCH1 mutations in dopa-responsive dystonia and Parkinson’s disease. J Neurol 265:1860–1870
Young CB, Sonne J (2018) Neuroanatomy, basal ganglia. StatPearls Publishing, Treasure Island
Yu YC, Kuo CL, Cheng WL, Liu CS, Hsieh M (2009) Decreased antioxidant enzyme activity and increased mitochondrial DNA damage in cellular models of Machado–Joseph disease. J Neurosci Res 87:1884–1891
Yu D, Sul JH, Tsetsos F, Nawaz MS, Huang AY, Zelaya I, Illmann C, Osiecki L, Darrow SM, Hirschtritt ME, Greenberg E, Muller-Vahl KR, Stuhrmann M, Dion Y, Rouleau G, Aschauer H, Stamenkovic M, Schlogelhofer M, Sandor P, Barr CL, Grados M, Singer HS, Nothen MM, Hebebrand J, Hinney A, King RA, Fernandez TV, Barta C, Tarnok Z, Nagy P, Depienne C, Worbe Y, Hartmann A, Budman CL, Rizzo R, Lyon GJ, McMahon WM, Batterson JR, Cath DC, Malaty IA, Okun MS, Berlin C, Woods DW, Lee PC, Jankovic J, Robertson MM, Gilbert DL, Brown LW, Coffey BJ, Dietrich A, Hoekstra PJ, Kuperman S, Zinner SH, Luethvigsson P, Saemundsen E, Thorarensen O, Atzmon G, Barzilai N, Wagner M, Moessner R, Ophoff R, Pato CN, Pato MT, Knowles JA, Roffman JL, Smoller JW, Buckner RL, Willsey AJ, Tischfield JA, Heiman GA, Stefansson H, Stefansson K, Posthuma D, Cox NJ, Pauls DL, Freimer NB, Neale BM, Davis LK, Paschou P, Coppola G, Mathews CA, Scharf JM (2019a) Interrogating the genetic determinants of Tourette’s syndrome and other tic disorders through genome-wide association studies. Am J Psychiatry 176:217–227
Yu XE, Gao S, Yang RM, Han YZ (2019b) MR imaging of the brain in neurologic Wilson disease. AJNR Am J Neuroradiol 40:178–183
Zetterberg H, van Swieten JC, Boxer AL, Rohrer JD (2019) Review: fluid biomarkers for frontotemporal dementias. Neuropathol Appl Neurobiol 45:81–87
Zhang L, McCarthy DM, Sharma N, Bhide PG (2015) Dopamine receptor and Galpha(olf) expression in DYT1 dystonia mouse models during postnatal development. PLoS One 10:e0123104
Zhong S, Wen S, Qiu Y, Yu Y, Xin L, He Y, Gao X, Fang H, Hong D, Zhang J (2019a) Bilateral striatal necrosis due to homoplasmic mitochondrial 3697G>A mutation presents with incomplete penetrance and sex bias. Mol Genet Genomic Med 7:e541
Zhong W, Huang Z, Tang X (2019b) A study of brain MRI characteristics and clinical features in 76 cases of Wilson’s disease. J Clin Neurosci 59:167–174
Zou L, Song Y, Zhou X, Chu J, Tang X (2019) Regional morphometric abnormalities and clinical relevance in Wilson’s disease. Mov Disord 34:545–554
Acknowledgements
The author thank Mr. E. Mitter-Ferstl, Ph.D., for secretarial and graphical work. The study was partially funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jellinger, K.A. Neuropathology and pathogenesis of extrapyramidal movement disorders: a critical update. II. Hyperkinetic disorders. J Neural Transm 126, 997–1027 (2019). https://doi.org/10.1007/s00702-019-02030-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02030-y