Abstract
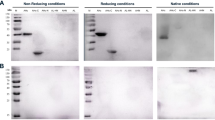
IncobotulinumtoxinA (Xeomin®) and onabotulinumtoxinA (BOTOX®) are unique botulinum neurotoxin type A (BoNT/A)-derived drugs. IncobotulinumtoxinA utilizes the naked 150 kDa holotoxin portion of BoNT/A, whereas onabotulinumtoxinA uses the complete native 900 kDa complex as drug substance. On the basis of purportedly similar pharmacological characteristics, these formulations were evaluated for potency by LD50 and mouse Digit Abduction Score (DAS) bioassays. DAS was also used to assess antigenicity. Full-range DAS dose–response profiles were achieved with four lots of each product, with similar observations between lots for a given product. Between products, however, the mean DAS potency of incobotulinumtoxinA (ED50 range 7.0–10.2 U/kg) was significantly lower than that of onabotulinumtoxinA (ED50 range 4.4–6.4 U/kg), consistent with lower measured potencies in the LD50 assay for incobotulinumtoxinA (potency range 62–82 U). In assessments of DAS duration of effect at similar unit doses, the observed lower potency of incobotulinumtoxinA translated into decreased peak efficacy and dose effect over time (i.e. shorter duration). In contrast, at equi-efficacious doses yielding near-maximal DAS responses, both toxin formulations were uniformly inhibited in a statistically significant manner when preincubated with rabbit-derived, onabotulinumtoxinA-neutralizing antibodies, supporting the position that inhibition of 150 kDa holotoxin serves as the common basis for neutralization and, therefore, incobotulinumtoxinA would not be expected to be effective in onabotulinumtoxinA-immunoresistant subjects (and vice versa). Further, with lower lot-to-lot relative potency, incobotulinumtoxinA is not dose-equivalent or interchangeable with onabotulinumtoxinA, suggesting that various aspects of drug product formulation may influence observed pharmacology.


Similar content being viewed by others
References
Aoki KR (2001) A comparison of the safety margins of botulinum neurotoxin serotypes A, B, and F in mice. Toxicon 39:1815–1820
Aoki KR (2005) Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 26:785–793
Aoki KR, Francis J (2011) Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Relat Disord 17(Suppl 1):S28–S33
Argoff CE (2002) A focused review on the use of botulinum toxins for neuropathic pain. Clin J Pain 18(6 Suppl):S177–S181
Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, Diener HC, Brin MF, PREEMPT 1 Chronic Migraine Study Group (2010) OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 30(7):793–803 (Epub 2010 Mar 17)
Benecke R (2009) Xeomin® in the treatment of cervical dystonia. Eur J Neurol 16(Suppl 2):6–10
Benecke R (2012) Clinical relevance of botulinum toxin immunogenicity. Biodrugs 26(2):e1–e9
Benecke R, Dressler D (2007) Botulinum toxin treatment of axial and cervical dystonia. Disabil Rehab 29(23):1769–1777
Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ (2010) Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache 50(9):1406–1418
Casale R, Tugnoli V (2008) Botulinum toxin for pain. Drugs R D 9:11–27
Cui M, Li Z, You S, Khanijou S, Aoki KR (2002) Mechanism of the antinociceptive effect of subcutaneous BOTOX: inhibition of peripheral and central nociceptive processing. Naunyn Schmiedebergs Arch Pharmacol 365(Suppl 2):R17
de Maio M (2008) Therapeutic uses of botulinum toxin: from facial palsy to autonomic disorders. Expert Opin Biol Ther 8:791–798
Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, Silberstein SD, Brin MF, PREEMPT 2 Chronic Migraine Study Group (2010) OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 30(7):804–814 (Epub 2010 Mar 17)
Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, Diener HC, Brin MF, PREEMPT Chronic Migraine Study Group (2010) OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 50(6):921–936 (Epub 2010 May 7)
Dolly JO, Aoki KR (2006) The structure and mode of action of different botulinum toxins. Eur J Neurol 13(Suppl 4):1–9
Dressler D (2009) Routine use of Xeomin® in patients previously treated with Botox®: long term results. Eur J Neurol 16(Suppl 2):2–5
Dressler D (2010) Comparing Botox® and Xeomin® for axillar hyperhydrosis. J Neural Transm 117:317–319
Dressler D (2012) Five-year experience with incobotulinumtoxinA (Xeomin®): the first botulinum toxin drug free of complexing proteins. Eur J Neurol 19(3):385–389 (Epub 2011 Oct 28)
Dressler D, Benecke R (2007) Pharmacology of therapeutic botulinum toxin preparations. Disabil Rehabil 29:1761–1768
Dressler D, Mander G, Fink K (2012) Measuring the potency labelling of onabotulinumtoxinA (Botox®) and incobotulinumtoxinA (Xeomin®) in an LD50 assay. J Neural Transm 119(1):13–15 (Epub 2011 Oct 5)
Frevert J (2009a) Xeomin®: an innovative new botulinum toxin type A. Eur J Neurol 16(Suppl 2):11–13
Frevert J (2009b) Xeomin is free from complexing proteins. Toxicon 54:697–701
Frevert J (2010) Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs RD 2(10):91–92
Frevert J, Dressler D (2010) Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biol Target Ther 4:325–332
Hunt T, Clarke K (2009) Potency evaluation of a formulated drug product containing 150-kD botulinum neurotoxin type A. Clin Neuropharmacol 32:28–31
Hunt T, Rupp D, Shimizu G, Tam K, Weidler J, Xie J (2010) Characterization of SNARE cleavage products generated by formulated botulinum neurotoxin type-A drug products. Toxins 2(8):2198–2212
Jankovic J (2009) Clinical efficacy and tolerability of Xeomin® in the treatment of blepharospasm. Eur J Neurol 16(Suppl 2):14–18
Jankovic J, Brin MF (1997) Botulinum toxin: historical perspective and potential new indications. Muscle Nerve Suppl 6:S129–S145
Jost WH (2007) Efficacy and safety of botulinum neurotoxin type A free of complexing proteins (NT 201) in cervical dystonia and blepharospasm. Future Neurol 2(5):485–493
Jost WH, Blümel J, Grafe S (2007) Botulinum neurotoxin type A free of complexing proteins (Xeomin®) in focal dystonia. Drugs 67(5):669–683
Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K (2009) Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol 32:213–218
Lietzow MA, Gielow ET, Le D, Zhang J, Verhagen MF (2008) Subunit stoichiometry of the Clostridium botulinum type A neurotoxin complex determined using denaturing capillary electrophoresis. Protein J 27:420–425
Luvisetto S, Marinelli S, Cobianchi S, Pavone F (2007) Anti-allodynic efficacy of botulinum neurotoxin A in a model of neuropathic pain. Neuroscience 145:1–4
Ney JP, Joseph KR (2007) Neurologic uses of botulinum neurotoxin type A. Neuropsychiatr Dis Treat 3:785–798
Park HJ, Lee Y, Lee J, Park C, Moon DE (2006) The effects of botulinum toxin A on mechanical and cold allodynia in a rat model of neuropathic pain. Can J Anaesth 53:470–477
Querama E, Fuglsang-Frederiksen A, Jensen TS (2010) The role of botulinum toxin in management of pain: an evidence-based review. Curr Opin Anaesthesiol 23:602–610
Roggenkämper P, Jost WH, Bihari K, Comes G, Grafe S (2006) Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm 113:303–312
Schulte-Mattler WJ (2008) Use of botulinum toxin A in adult neurological disorders: efficacy, tolerability and safety. CNS Drugs 22:725–738
Simpson L (2004) Identification of the major steps in botulinum toxin action. Ann Rev Pharmacol Toxicol 44:167–193
Singh SK (2011) Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci 100(2):354–387
Thant ZS and Tan EK (2003) Emerging therapeutic applications of botulinum toxin. Med Sci Monit 9(2):RA40–RA48
Wohlfarth K, Müller C, Sassin I, Comes G, Grafe S (2007) Neurophysiological double-blind trial of a botulinum neurotoxin type A free of complexing proteins. Clin Neuropharmacol 30:86–94
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Brown, M., Nicholson, G., Ardila, M.C. et al. Comparative evaluation of the potency and antigenicity of two distinct BoNT/A-derived formulations. J Neural Transm 120, 291–298 (2013). https://doi.org/10.1007/s00702-012-0854-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0854-3