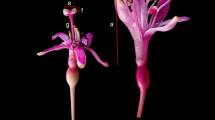
Abstract
Documenting nonhermaphroditic sexual systems, a task of major interest for evolutionary biology, is particularly problematic in rare or remote species, for which field sampling is difficult or field specimens or herbarium material is scarce. In addition to hermaphroditism, monoecy and dioecy have been reported in the family Sapotaceae. Nevertheless, the sexual system of some New Caledonian taxa currently included in the genus Planchonella remains vaguely characterized as having “bisexual and female flowers.” In the present study we investigate the significance of female flowers in Planchonella endlicheri, P. laetevirens, and P. latihila. We confirmed that P. endlicheri and P. laetevirens are gynomonoecious in nature, and that P. latihila is gynomonoecious at least when growing in a greenhouse. In addition, we found sexual dimorphism in floral size in P. endlicheri, namely a lower corolla length in female compared to bisexual flowers. Two kinds of position effects on floral sex were present in P. endlicheri. At the twig level, upper flowers had an increased probability of being female and at the inflorescence (fascicle) level, central flowers were predominantly female while lateral flowers were mainly bisexual. Our study illustrates how observational studies on rare or remote species can improve our knowledge of sexual systems in plants and document relevant evolutionary patterns in sexual dimorphism and position effects of floral sex.




Similar content being viewed by others
References
Aubréville A (1961a) Notes sur des Poutériées américaines. Adansonia 1:150–191
Aubréville A (1961b) Notes sur les Sapotacées africaines et sud-américaines. Adansonia 1:6–38
Aubréville A (1963) Notes sur des Sapotacées africaines. Adansonia 3:227–231
Aubréville A (1967) Flore de la Nouvelle-Calédonie et dépendances. I. Sapotacées. Muséum National d’Histoire Naturelle, Paris
Baker HG (1948) Corolla-size in gynodioecious and gynomonoecious species of flowering plants. Proc Leeds Phil Lit Soc 5:136–139
Bawa KS, Beach JH (1981) Evolution of sexual systems in flowering plants. Ann Missouri Bot Gard 68:254–274
Bernardello G, Anderson GJ, López P, Cleland MA, Stuessy TF, Crawford DJ (1999) Reproductive biology of Lactoris fernandeziana (Lactoridaceae). Am J Bot 86:829–840
Bertin RI (1993) Incidence of monoecy and dichogamy in relation to self-fertilization in angiosperms. Am J Bot 80:557–560
Black L, Bukovac MJ, Stopar M (2000) Intraspur fruit competition and position influence fruit size at harvest and response to chemical thinning agents in spur-type ‘Delicious’ apple. Acta Horti 527:119–125
Burtt BL (1977) Aspects of diversification in the capitulum. In: Heywood VH, Harborne JB (eds) The biology and chemistry of the Compositae, vol I. Academic Press, London, pp 41–59
Charlesworth D (1985) Distribution of dioecy and self-incompatibility in angiosperms. In: Greenwood PJ, Harvey PH, Slatkin M (eds) Evolution: essays in honour of John Maynard Smith. Cambridge University Press, Cambridge, pp 237–268
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Clayton WD, Renvoize SA (1986) Genera graminum: grasses of the world. Royal Botanic Gardens, Kew
Condon MA, Gilbert LE (1988) Sex expression of Gurania and Psiguria (Cucurbitaceae): Neotropical vines that change sex. Am J Bot 75:875–884
Delph LF, Galloway LF, Stanton ML (1996) Sexual dimorphism in flower size. Am Nat 148:299–320
Dem’yanova AEI (1985) Distribution of gynodioecy in flowering plants. Bot Zhur 70:1289–1301
Diggle PK (2003) Architectural effects on floral form and function: a review. In: Stuessy T, Hörandl E, Mayer V (eds) Deep morphology: toward a renaissance of morphology in plant systematics. Koeltz, Königstein, pp 63–80
Eckhart VM (1999) Sexual dimorphism in flowers and inflorescences. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 123–148
El-Keblawy A, Lovett-Doust J, Lovett-Doust L, Shaltout KH (1995) Labile sex expression and dynamics of gender in Thymelaea hirsuta. Ecoscience 2:55–66
Elle E, Meagher TR (2000) Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. Am Nat 156:622–636
Emms SK (1993) Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. Am J Bot 80:914–923
Givnish TJ (1982) Outcrossing versus ecological constraints in the evolution of dioecy. Am Nat 119:849–865
Grayum MH (1990) Evolution and phylogeny of the Araceae. Ann Missouri Bot Gard 77:628–697
Heslop-Harrison J (1957) The experimental modification of sex expression in flowering plants. Biol Rev 32:38–90
Heslop-Harrison J (1972) Sexuality of angiosperms. In: Steward FC (ed) Plant physiology: a treatise. Vol. VI C: physiology of development: from seeds to sexuality. Academic Press, New York, pp 133–289
Huang S-Q (2003) Flower dimorphism and the maintenance of andromonoecy in Sagittaria guyanensis ssp. lappula (Alismataceae). New Phytol 157:357–364
Ishii HS (2004) Increase of male reproductive components with size in an animal-pollinated hermaphrodite, Narthecium asiaticum (Liliaceae). Funct Ecol 18:130–137
Kaul V, Sharma N, Koul AK (2002) Reproductive effort and sex allocation strategy in Commelina benghalensis L., a common monsoon weed. Bot J Linn Soc 140:403–413
Manicacci D, Després L (2001) Male and hermaphrodite flowers in the alpine lily Lloydia serotina. Can J Bot 79:1107–1114
Mayer SS, Charlesworth D (1991) Cryptic dioecy in flowering plants. Trends Ecol Evol 6:320–325
McKone MJ, Ostertag R, Rauscher JT, Heiser DA, Russell FL (1995) An exception to Darwin’s syndrome: floral position, protogyny, and insect visitation in Besseya bullii (Scrophulariaceae). Oecologia 101:68–74
Munzinger J, Swenson U (2009) Three new species of Planchonella (Sapotaceae) with a dichotomous and an online key to the genus in New Caledonia. Adansonia 31:175–189
O’Brien SP (1994) Andromonoecy and fruit set in Leptospermum myrsinoides and L. continentale (Myrtaceae). Aust J Bot 42:751–762
Parfitt BD (1985) Dioecy in North American Cactaceae: a review. SIDA 11:200–206
Pennington TD (1990) Sapotaceae. Flora Neotrop 52:1–770
Pennington TD (2004) Sapotaceae. In: Kubitzki K (ed) The families and genera of vascular plants, vol 6. Springer, Berlin, pp 390–421
Poppendieck H-H (1987) Monoecy and sex changes in Freycinetia (Pandanaceae). Ann Missouri Bot Gard 74:314–320
Primack RB, Lloyd DG (1980) Andromonoecy in the New Zealand montane shrub manuka, Leptospermum scoparium (Myrtaceae). Am J Bot 67:361–368
Renner SS, Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. Am J Bot 82:596–606
Sakai AK, Weller SG (1999) Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin, pp 1–31
Sakai AK, Wagner WL, Ferguson DM, Herbst DR (1995) Origins of dioecy in the Hawaiian flora. Ecology 76:2517–2529
Sawyer NW, Anderson GJ (2000) Dioecy in South American Deprea (Solanaceae). Biotropica 32:291–298
Solomon BP (1986) Sexual allocation and andromonoecy: resource investment in male and hermaphroditic flowers of Solanum carolinense (Solanaceae). Am J Bot 73:1215–1221
Spalik K, Woodell SRJ (1994) Regulation of pollen production in Anthriscus sylvestris, an andromonoecious species. Int J Plant Sci 155:750–754
Swenson U, Munzinger J, Bartish I (2007a) Molecular phylogeny of Planchonella (Sapotaceae) and eight new species from New Caledonia. Taxon 56:329–354
Swenson U, Bartish I, Munzinger J (2007b) Phylogeny, diagnostic characters, and generic limitation of Australasian Chrysophylloideae (Sapotaceae, Ericales): evidence from ITS sequence data and morphology. Cladistics 23:201–228
Thompson PN, Gornall RJ (1995) Breeding systems in Coriaria (Coriariaceae). Bot J Linn Soc 117:293–304
Thomson JD, Barrett SCH (1981) Selection for outcrossing, sexual selection, and the evolution of dioecy in plants. Am Nat 118:443–449
Ueno S, Kadono Y (2001) Monoecious plants of Myriophyllum ussuriense (Regel) Maxim. in Japan. J Plant Res 114:375–376
Ushimaru A, Itagaki T, Ishii HS (2003a) Floral correlations in an andromonoecious species, Commelina communis (Commelinaceae). Plant Species Biol 18:103–106
Ushimaru A, Itagaki T, Ishii HS (2003b) Variation in floral organ size depends on function: a test with Commelina communis, an andromonoecious species. Evol Ecol Res 5:615–622
Utteridge TMA, Saunders RMK (2001) Sexual dimorphism and functional dioecy in Maesa perlarius and M. japonica (Maesaceae/Myrsinaceae). Biotropica 33:368–374
Wise MJ, Coffey LE, Abrahamson WG (2008) Nutrient stress and gall flies interact to affect floral-sex ratio in gynomonoecious Solidago altissima. Am J Bot 95:1233–1239
Yampolsky C, Yampolsky H (1922) Distribution of sex forms in the phanerogamic flora. Bibl Genet 3:1–62
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Upper Saddle River
Acknowledgments
M.M. thanks Ulf Swenson for first pointing to the presence of female flowers in Planchonella and for advice on the study species. We thank Patrick Taton (Koghi Parc Aventure) for access to P. endlicheri within their property, Maeva Guyomar for help with the field work, and Laure Barrabé and Gilles Dagostini (IRD-NOU) and Marion Beauvallet (CIRAD) for collecting fallen corollas. Gildas Gateblé (SRMH-IAC) kindly provided us corollas from P. latihila cuttings. South Province’s Direction de l’Environnement (DENV) provided collecting permits. Planchonella endlicheri is studied as a plant model for the ANR ULTRABIO program research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Méndez, M., Munzinger, J. Planchonella, first record of gynomonoecy for the family Sapotaceae. Plant Syst Evol 287, 65–73 (2010). https://doi.org/10.1007/s00606-010-0290-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-010-0290-5