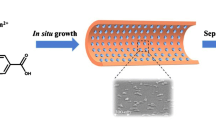
Abstract
Two-dimensional (2D) COFs have been successfully applied for various applications, such as capillary electrochromatography (CEC). Compared with 2D COFs, three-dimensional (3D) COFs have higher surface area and lower density, which should have superior potential as the separation medium in CEC. However, the 3D COFs on the inner wall of capillary is hard to fabricate in situ. Up to date, the application of 3D COFs in open-tubular capillary electrochromatography (OT-CEC) is still considered a challenge. For the first time the COF-300-coated capillary was prepared by in situ growth (COF-300 was made from terephthalaldehyde and tetra-(4-anilyl)-methane) on OT-CEC. Benzene, methylbenzene, styrene, ethylbenzene, naphthalene, 1-methylnaphthalene, and propylbenzene were used to evaluate the performance of the COF-300-coated capillary by OT-CEC. For three consecutive runs, the intraday relative standard deviations (RSDs) of migration time and peak areas were 0.1–0.4% and 2.5–8.3%, respectively. The interday RSDs of migration time and peak areas were 0.2–0.5% and 1.0–10.8%, respectively. Five groups of aromatic co mpounds were used to further study the separation mechanism, which indicated that hydrophobic interaction and size selection interaction are the main factors. It should be noted that the COF-300-coated capillary can be used for more than 140 runs with no observable changes of the separation efficiency.

The 3D COF-300-coated capillary was prepared by in situ growth for OT-CEC. Six groups of aromatic compounds were separated by 3D COF-300-coated capillary. Size selection and hydrophobic interaction affect the migration time of analytes.






Similar content being viewed by others
References
Cote AP, Benin AI, Ockwig NW, O'Keeffe M, Matzger AJ, Yaghi OM (2005) Porous, crystalline, covalent organic frameworks. Science 310(5751):1166–1170
El-Kaderi HM, Hunt JR, Mendoza-Cortés JL, Côté AP, Taylor RE, O'Keeffe M, Yaghi OM (2007) Designed synthesis of 3D covalent organic frameworks. Science 316(5822):268–272
Feng X, Ding X, Jiang D (2012) Covalent organic frameworks. Chem Soc Rev 41(18):6010–6022
Ding S, Wang W (2013) Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev 42(2):548–568
Han SS, Furukawa H, Yaghi OM, Goddard Iii WA (2008) Covalent organic frameworks as exceptional hydrogen storage materials. J Am Chem Soc 130(35):11580–11581
Furukawa H, Yaghi OM (2009) Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J Am Chem Soc 131(25):8875–8883
Ding S, Gao J, Wang Q, Zhang Y, Song W, Su C, Wang W (2011) Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J Am Chem Soc 133(49):19816–19822
Xu H, Chen X, Gao J, Lin J, Addicoat M, Irle S, Jiang D (2014) Catalytic covalent organic frameworks via pore surface engineering. Chem Commun 50(11):1292–1294
Wan S, Guo J, Kim J, Ihee H, Jiang D (2008) A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew Chem Int Ed 47(46):8826–8830
Wan S, Gándara F, Asano A, Furukawa H, Saeki A, Dey SK, Liao L, Ambrogio MW, Botros YY, Duan X, Seki S, Stoddart JF, Yaghi OM (2011) Covalent organic frameworks with high charge carrier mobility. Chem Mater 23(18):4094–4097
DeBlase CR, Silberstein KE, Truong T-T, Abruña HD, Dichtel WR (2013) β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J Am Chem Soc 135(45):16821–16824
Fang Q, Wang J, Gu S, Kaspar RB, Zhuang Z, Zheng J, Guo H, Qiu S, Yan Y (2015) 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J Am Chem Soc 137(26):8352–8355
Yang C, Liu C, Cao Y, Yan X (2015) Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem Commun 51(61):12254–12257
Niu X, Ding S, Wang W, Xu Y, Xu Y, Chen H, Chen X (2016) Separation of small organic molecules using covalent organic frameworks-LZU1 as stationary phase by open-tubular capillary electrochromatography. J Chromatogr A 1436:109–117
Bao T, Tang P, Kong D, Mao Z, Chen Z (2016) Polydopamine-supported immobilization of covalent-organic framework-5 in capillary as stationary phase for electrochromatographic separation. J Chromatogr A 1445:140–148
Qian H, Yang C, Yan X (2016) Bottom-up synthesis of chiral covalent organic frameworks and their bound capillaries for chiral separation. Nat Commun 7:12104–12112
Kong D, Bao T, Chen Z (2017) In situ synthesis of the imine-based covalent organic framework LZU1 on the inner walls of capillaries for electrochromatographic separation of nonsteroidal drugs and amino acids. Microchim Acta 4(184):1169–1176
Zhao L, Lv W, Niu X, Pan C, Chen H, Chen X (2019) An azine-linked covalent organic framework as stationary phase for separation of environmental endocrine disruptors by open-tubular capillary electrochromatography. J Chromatogr A:460722. https://doi.org/10.1016/j.chroma.2019.460722
Xu Y, Niu X, Dong Y, Zhang H, Li X, Chen H, Chen X (2013) Preparation and characterization of open-tubular capillary column modified with graphene oxide nanosheets for the separation of small organic molecules. J Chromatogr A 1284:180–187
Xu Y, Xu L, Qi S, Dong Y, Rahman Z, Chen H, Chen X (2013) In situ synthesis of MIL-100(Fe) in the capillary column for capillary electrochromatographic separation of small organic molecules. Anal Chem 85(23):11369–11375
Xu Y, Lv W, Ren C, Niu X, Chen H, Chen X (2018) In situ preparation of multilayer coated capillary column with HKUST-1 for separation of neutral small organic molecules by open tubular capillary electrochromatography. J Chromatogr A 1532:223–231
Zhang J, Zhu P, Xie S, Zi M, Yuan L (2018) Homochiral porous organic cage used as stationary phase for open tubular capillary electrochromatography. Anal Chim Acta 999:169–175
Sun X, Tao Y, Du Y, Ding W, Chen C, Ma X (2019) Metal organic framework HKUST-1 modified with carboxymethyl-β-cyclodextrin for use in improved open tubular capillary electrochromatographic enantioseparation of five basic drugs. Microchim Acta 186(7):462–469
Li Z, Mao Z, Chen Z (2019) Polydopamine-assisted immobilization of a zinc(II)-derived metal-organic cage as a stationary phase for open-tubular capillary electrochromatography. Microchim Acta 186(7):449–457
Li Z, Mao Z, Chen Z (2019) In-situ growth of a metal organic framework composed of zinc(II), adeninate and biphenyldicarboxylate as a stationary phase for open-tubular capillary electrochromatography. Microchim Acta 186(2):53–60
Torres-Knoop A, Heinen J, Krishna R, Dubbeldam D (2015) Entropic separation of styrene/ethylbenzene mixtures by exploitation of subtle differences in molecular configurations in ordered crystalline nanoporous adsorbents. Langmuir 31(12):3771–3778
Cao B, Henson MA (2002) Modeling of spiral wound pervaporation modules with application to the separation of styrene/ethylbenzene mixtures. J Membr Sci 197(1):117–146
Huang J, Han X, Yang S, Cao Y, Yuan C, Liu Y, Wang J, Cui Y (2019) Microporous 3D covalent organic frameworks for liquid chromatographic separation of xylene isomers and ethylbenzene. J Am Chem Soc 141(22):8996–9003
Hartlieb KJ, Holcroft JM, Moghadam PZ, Vermeulen NA, Algaradah MM, Nassar MS, Botros YY, Snurr RQ, Stoddart JF (2016) CD-MOF: a versatile separation medium. J Am Chem Soc 138(7):2292–2301
Qian H, Yang C, Yan X (2018) Layer-by-layer preparation of 3D covalent organic framework/silica composites for chromatographic separation of position isomers. Chem Commun 54(83):11765–11768
Uribe-Romo FJ, Hunt JR, Furukawa H, Klöck C, O’Keeffe M, Yaghi OM (2009) A crystalline imine-linked 3-D porous covalent organic framework. J Am Chem Soc 131(13):4570–4571
Fu J, Das S, Xing G, Ben T, Valtchev V, Qiu S (2016) Fabrication of COF-MOF composite membranes and their highly selective separation of H2/CO2. J Am Chem Soc 138(24):7673–7680
Luo Z, Wang S, Zhou L, Hu Z (2008) Silanizing agent for semipermanent wall coating in micellar electrokinetic capillary chromatography. Talanta 76(2):413–418
Dementjev A, De Graaf A, Van de Sanden M, Maslakov K, Naumkin A, Serov A (2000) X-ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam Relat Mater 9(11):1904–1907
Diao X, Zhang F, Yang B, Liang X, Ke Y, Chu X (2012) Preparation and evaluation of C10-cationic latex particle coated open-tubular column for capillary electrochromatography. J Chromatogr A 1267:127–130
Funding
The authors are grateful for financial support from the National Natural Science Foundation of China (NOs. 21675068, 21705064).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.13 mb).
Rights and permissions
About this article
Cite this article
Niu, X., Lv, W., Sun, Y. et al. In situ fabrication of 3D COF-300 in a capillary for separation of aromatic compounds by open-tubular capillary electrochromatography. Microchim Acta 187, 233 (2020). https://doi.org/10.1007/s00604-020-4196-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4196-9