Abstract
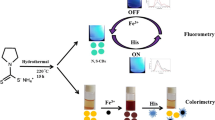
Nitrogen, sulfur, phosphorus, and chlorine co-doped carbon nanodots (NSPCl-CNDs) were fabricated by acid-base neutralization and exothermic carbonization of glucose. The obtained NSPCl-CNDs possess excellent fluorescence properties and good biocompatibility. Curcumin (Cur) can dramatically quench the fluorescence of NSPCl-CNDs based on a synergistic effect of electrostatic interaction, inner filter effect, and static quenching, so a “turn-off” fluorescent probe for Cur detection was constructed with linear ranges of 0.24–13.16 μM and 13.62–57.79 μM. The LOD and LOQ of this fluorescent probe for Cur are 8.71 nM and 29.03 nM, respectively. More importantly, the fluorescence of the NSPCl-CNDs-Cur system can be recovered by europium ion (Eu3+), so a “turn-on” fluorescent probe for Eu3+ determination was established. The linear range, LOD, and LOQ for the detection of Eu3+ were 2.36–32.91 μΜ, 73.29 nM, and 244.30 nM, respectively. The proposed fluorescence methods were successfully utilized for Cur and Eu3+ determination in real samples with recoveries in the range 95.64–104.13% and 97.06–98.70%, respectively. Furthermore, the qualitative analysis of Cur can be realized by reagent strips with satisfying results. Finally, the as-constructed “off-on” fluorescent probe was successfully used to sequentially analyze Cur and Eu3+ at the cellular level. This method is simple and easy to implement, manifesting that NSPCl-CNDs have potential application value in fluorescent probing, food and drug testing, environmental monitoring, and cellular labeling.

Graphical abstract









Similar content being viewed by others
References
Prasad S, Tyagi AK, Aggarwal BB (2014) Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat 46:2–18
Esatbeyoglu T, Huebbe P, Ernst IMA, Chin D, Wagner AE, Rimbach G (2012) Curcumin-from molecule to biological function. Angew Chem Int Ed 51:5308–5332
Chan W-H, Wu H-Y, Chang WH (2006) Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem Toxicol 44:1362–1371
Esther P-P, Soriano ML, Gema MD, Eulogio JL-M, Ana MC, Ángel R (2020) Discrimination between nanocurcumin and free curcumin using graphene quantum dots as a selective fluorescence probe. Microchim Acta 187:446
Bu L, Luo T, Peng H, Li L, Long D, Peng J, Huang J (2019) One-step synthesis of N-doped carbon dots, and their applications in curcumin sensing, fluorescent inks, and super-resolution nanoscopy. Microchim Acta 186:675
Zhao M, Qi W, Fu Y, He H, Wu D, Qi L, Li R (2019) Electrochemiluminescence “turn-off” detection of curcumin via energy transfer using luminol-doped silica nanoparticles. Microchim Acta 186:409
Liu L, Hua R, Zhang X, Li X, Zhang W, Qi X, Zhang T, Yang S, Zhang H, Liang H (2020) Spectral identification and detection of curcumin based on lanthanide upconversion nanoparticles. Appl Surf Sci 525:146566
Yang H, Li X, Wang X, Chen W, Bian W, Choi MMF (2018) Silver-doped graphite carbon nitride nanosheets as fluorescent probe for the detection of curcumin. Luminescence 33:1062–1069
Yang R, Mu W-Y, Chen Q-Y (2019) Urazole-Au nanocluster as a novel fluorescence probe for curcumin determination and mitochondria imaging. Food Anal Methods 12:1805–1812
Yu M, Lin J, Fang J (2005) Silica spheres coated with YVO4:Eu3+ layers via sol−gel process: a simple method to obtain spherical core−shell phosphors. Chem Mater 17:1783–1791
Lehmann O, Kömpe K, Haase M (2004) Synthesis of Eu3+-doped core and core/shell nanoparticles and direct spectroscopic identification of dopant sites at the surface and in the interior of the particles. J Am Chem Soc 126:14935–14942
González V, Vignati DAL, Leyval C, Giamberini L (2014) Environmental fate and ecotoxicity of lanthanides: are they a uniform group beyond chemistry? Environ Int 71:148–157
Li C, Zhuang Z, Huang F, Wu Z, Hong Y, Lin Z (2013) Recycling rare earth elements from industrial wastewater with flowerlike nano-Mg(OH)2. ACS Appl Mater Interfaces 5:9719–9725
Bruzzoniti MC, Mentasti E, Sarzanini C, Braglia M, Cocito G, Kraus J (1996) Determination of rare earth elements by ion chromatography. Separation procedure optimization. Anal Chim Acta 322:49–54
Verplanck PL, Antweiler RC, Nordstrom DK, Taylor HE (2001) Standard reference water samples for rare earth element determination. Appl Geochem 16:231–244
Itoh H, Hachiya H, Tsuchiya M, Suzuki Y, Asano Y (1984) Determination of solubility products of rare earth fluorides by fluoride ion-selective electrode. Bull Chem Soc Jpn 57:1689–1690
Pasinli T, Eroglu AE, Shahwan T (2005) Preconcentration and atomic spectrometric determination of rare earth elements (REEs) in natural water samples by inductively coupled plasma atomic emission spectrometry. Anal Chim Acta 547:42–49
Siriraks A, Kingston HM, Riviello JM (1990) Chelation ion chromatography as a method for trace elemental analysis in complex environmental and biological samples. Anal Chem 62:1185–1193
Crozaz G, Zinner E (1985) Ion probe determinations of the rare earth concentrations of individual meteoritic phosphate grains. Earth Planet Sci Lett 73:41–52
Ma X, Xu Z, Yuan H, He Y, Xiao D, Choi MMF (2010) High-sensitive and selective Eu3+ electrochemical sensor based on LaB6 electrode and sodium dodecylbenzene sulfonate. Sens Actuators B-Chem 147:152–158
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Elec-trophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737
Kang Z, Lee S-T (2019) Carbon dots: advances in nanocarbon applications. Nanoscale 11:19214–19224
Wang Y, Hu A (2014) Carbon quantum dots: synthesis, properties and applications. J Mater Chem C 2:6921–6939
Cui X, Zhu L, Wu J, Hou Y, Wang P, Wang Z, Yang M (2014) A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens Bioelectron 63:506–512
Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun 48:8835–8837
Hola K, Zhang Y, Wang Y, Giannelis EP, Zboril R, Rogach AL (2014) Carbon dots-emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 9:590–603
Hettiarac S, Graham R, Mintz KJ, Zhou Y, Vanni S, Peng Z, Leblanc RM (2019) Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 11:6192–6205
Xu Y, Wu M, Liu Y, Feng X-Z, Yin X-B, He X-W, Zhang Y-K (2013) Nitrogen-doped carbon dots: a facile and general preparation method, photoluminescence investigation, and imaging applications. Chemistry 19:2276–2283
Wang Z-X, Yu X-H, Li F, Kong F-Y, Lv W-X, Fan D-H, Wang W (2017) Preparation of boron-doped carbon dots for fluorometric determination of Pb(II), Cu(II) and pyrophosphate ions. Microchim Acta 184:4775–4783
Qian Z, Shan X, Chai L, Ma J, Chen J, Feng H (2014) Si-doped carbon quantum dots: a facile and general preparation strategy, bioimaging application, and multifunctional sensor. ACS Appl Mater Interfaces 6:6797–6805
Zou S, Hou C, Fa H, Zhang L, Ma Y, Dong L, Li D, Huo D, Yang M (2017) An efficient fluorescent probe for fluazinam using N, S co-doped carbon dots from L-cysteine. Sens Actuators B-Chem 239:1033–1041
Rong M-C, Zhang K-X, Wang Y-R, Chen X (2017) The synthesis of B, N-carbon dots by a combustion method and the application of fluorescence detection for Cu2+. Chinese Chem Lett 28:1119–1124
Gong Y, Yu B, Yang W, Zhang XL (2016) Phosphorus, and nitrogen co-doped carbon dots as a fluorescent probe for real-time measurement of reactive oxygen and nitrogen species inside macrophages. Biosens Bioelectron 79:822–828
Wang C, Sun D, Zhuo K, Zhang H, Wang J (2014) Simple and green synthesis of nitrogen-, sulfur-, and phosphorus-co-doped carbon dots with tunable luminescence properties and sensing application. RSC Adv 4:54060–54065
Wu B, Liu X, Shi X, Han W, Wang C, Jiang L (2019) Highly photoluminescent and temperature-sensitive P, N, B-co-doped carbon quantum dots and their highly sensitive recognition for curcumin. RSC Adv 9:8340–8349
Gong X, Lu W, Paau MC, Hu Q, Wu X, Shuang S, Dong C, Choi MMF (2015) Facile synthesis of nitrogen-doped carbon dots for Fe3+ sensing and cellular imaging. Anal Chim Acta 861:74–84
Gong X, Zhang Q, Gao Y, Shuang S, Choi MMF, Dong C (2016) Phosphorus and nitrogen dual-doped hollow carbon dot as a nanocarrier for doxorubicin delivery and biological imaging. ACS Appl Mater Interfaces 8:11288–11297
Gong X, Wang H, Liu Y, Hu Q, Gao Y, Yang Z, Shuang S, Dong C (2019) A di-functional and label-free carbon-based chem- nanosensor for real-time monitoring of pH fluctuation and quantitative determining of curcumin. Anal Chim Acta 1057:132–144
Wang H, Zhang L, Guo X, Dong W, Wang R, Shuang S, Gong X, Dong C (2019) Comparative study of Cl,N-Cdots and N-Cdots and application for trinitrophenol and ClO sensor and cell-imaging. Anal Chim Acta 1091:76–87
Tillborg H, Nilsson A, Hernnäs B, Mårtensson N, Palmer RE (1993) X-ray and UV photoemission studies of mono-, bi- and multilayers of physisorbed molecules: O2 and N2 on graphite. Surf Sci 295:1–12
Sodhi RNS, Cavell RG (1986) KLL auger and core level (1s and 2p) photoelectron shifts in a series of gaseous sulfur compounds. J Electron Spectrosc Relat Phemon 41:1–24
Gong X, Hu Q, Paau MC, Zhang Y, Shuang S, Dong C, Choi MMF (2014) Red-green-blue fluorescent hollow carbon nanoparticles isolated from chromatographic fractions for cellular imaging. Nanoscale 6:8162–8170
Yan CX, Cavell RG (1987) Gas phase phosphorus (1s and 2p) binding energy and auger shifts in some polarizable phosphorus compoinds. J Electron Spectrosc Rela Phemon 42:49–60
Waltman RJ, Pacansky J, Bates CW (1993) X-ray photoelectron spectroscopic studies on organic photoconductors: evaluation of atomic charges on chlorodiane blue and p-(diethylamino)benzaldehyde diphenylhydrazone. Chem Mater 5:1799–1804
Chang CM (1993) High resolution XPS of organic polymers: the scienta ESCA300 database (Beamson, G.; Briggs, D.). J Chem Educ 70:A25
Hu Q, Gao L, Rao S-Q, Yang Z-Q, Li T, Gong X (2019) Nitrogen and chlorine dual-doped carbon nanodots for determination of curcumin in food matrix via inner filter effect. Food Chem 280:195–202
Baig MMF, Chen Y-C (2017) Bright carbon dots as fluorescence sensing agents for bacteria and curcumin. J Colloid Interface Sci 501:341–349
Song Y, Zhu S, Xiang S, Zhao X, Zhang J, Zhang H, Fu Y, Yang B (2014) Investigation into the fluorescence quenching behaviors and applications of carbon dots. Nanoscale 6:4676–4682
Zhao J, Zhao G, Liang H, Zhang H (2005) Fluorescence determination of europium with pyridine-2,4,6-tricarboxylicacid. Rare Metals 24:115–118
Yang C, Huang H (2003) Fluorimetric determination of europium, terbium and dysprosium with orotic or isoorotic acid. Chinese J Anal Chem 31:1079–1081
Liawruangrath S, Sakulkhaemaruethai S (2003) Flow injection spectrophotometric determination of europium using chlortetracycline. Talanta 59:9–18
Bassett AP, Magennis SW, Glover PB, Lewis DJ, Spencer N, Parsons S, Williams RM, Cola LD, Pikramenou Z (2004) Highly luminescent, triple- and quadruple-stranded, dinuclear Eu, Nd, and Sm(III) lanthanide complexes based on bis-diketonate ligands. J Am Chem Soc 126:9413–9424
Song Y-M, Xu J-P, Ding L, Hou Q, Liu J-W, Zhu Z-L (2009) Syntheses, characterization and biological activities of rare earth metal complexes with curcumin and 1,10-phenanthroline-5,6-dione. J Inorg Biochem 103:396–400
Funding
This work was supported by the National Natural Science Foundation of China (NO. 21705101), Shanxi Provincial Key Research and Development Project (201903D121109), China Postdoctoral Science Foundation (No. 2018 M642969), and Natural Science Foundation of Shanxi Province (No. 201801D121040).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2993 kb)
Rights and permissions
About this article
Cite this article
Hao, Y., Wang, H., Wang, Z. et al. Nitrogen, sulfur, phosphorus, and chlorine co-doped carbon nanodots as an “off-on” fluorescent probe for sequential detection of curcumin and europium ion and luxuriant applications. Microchim Acta 188, 16 (2021). https://doi.org/10.1007/s00604-020-04618-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04618-8