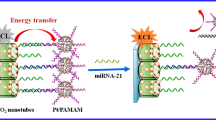
Abstract
A novel “off-on” electrochemiluminescence (ECL) aptasensor based on ECL resonance energy transfer (RET) system from 3,4,9,10-perylenetetracar-boxylic acid/the metal-organic frameworks NH2-MIL-125(Ti) (PTCA/NH2-MIL-125) to Au nanoparticles (Au NPs) was put forward for microcystin-LR (MC-LR) assay. The electrochemiluminescence (ECL) emission spectra of PTCA/NH2-MIL-125 show excellent spectral overlap with the UV-vis absorption spectrum of Au NPs. A linear response ranging from 10 fM (9.95 × 10−6 μg L−1) to 0.1 μM (99.5 μg L−1) was obtained, and the detection limit was 3.6 fM (3.58 × 10−6 μg L−1) under optimal conditions at a potential of − 1.6 V (S/N = 3). Besides, the prepared ECL-RET MC-LR aptasensor exhibited good selectivity and stability.

Graphical abstract






Similar content being viewed by others
References
Du X, Jiang D, Li H, Hao N, You T, Wang K (2018) An intriguing signal-off responsive photoelectrochemical aptasensor for ultrasensitive detection of microcystin-LR and its mechanism study. Sensors Actuators B Chem 259:316–324. https://doi.org/10.1016/j.snb.2017.12.065
Zhang J-J, Kang T-F, Hao Y-C, Lu L-P, Cheng S-Y (2015) Electrochemiluminescent immunosensor based on CdS quantum dots for ultrasensitive detection of microcystin-LR. Sensors Actuators B Chem 214:117–123. https://doi.org/10.1016/j.snb.2015.03.019
Du X, Jiang D, Dai L, Zhou L, Hao N, Qian J, Qiu B, Wang K (2016) Fabricating photoelectrochemical aptasensor for selectively monitoring microcystin-LR residues in fish based on visible light-responsive BiOBr nanoflakes/N-doped graphene photoelectrode. Biosens Bioelectron 81:242–248. https://doi.org/10.1016/j.bios.2016.02.072
Zhang W, Han C, Jia B, Saint C, Nadagouda M, Falaras P, Sygellou L, Vogiazi V, Dionysiou DD (2017) A 3D graphene-based biosensor as an early microcystin-LR screening tool in sources of drinking water supply. Electrochim Acta 236:319–327. https://doi.org/10.1016/j.electacta.2017.03.161
Lotierzo M, Abuknesha R, Davis F, Tothill IE (2012) A membrane-based ELISA assay and electrochemical immunosensor for microcystin-LR in water samples. Environ Sci Technol 46(10):5504–5510. https://doi.org/10.1021/es2041042
Li Y-W, Zhan X-J, Xiang L, Deng Z-S, Huang B-H, Wen H-F, Sun T-F, Cai Q-Y, Li H, Mo C-H (2014) Analysis of trace microcystins in vegetables using solid-phase extraction followed by high performance liquid chromatography triple-quadrupole mass spectrometry. J Agric Food Chem 62(49):11831–11839. https://doi.org/10.1021/jf5033075
Xu C, Yang Y, Liu L, Li J, Liu X, Zhang X, Liu Y, Zhang C, Liu X (2018) Microcystin-LR nanobody screening from an alpaca phage display nanobody library and its expression and application. Ecotoxicol Environ Saf 151:220–227. https://doi.org/10.1016/j.ecoenv.2018.01.003
Taghdisi SM, Danesh NM, Ramezani M, Ghows N, Mousavi Shaegh SA, Abnous K (2017) A novel fluorescent aptasensor for ultrasensitive detection of microcystin-LR based on single-walled carbon nanotubes and dapoxyl. Talanta 166:187–192. https://doi.org/10.1016/j.talanta.2017.01.053
Chinnappan R, AlZabn R, Abu-Salah KM, Zourob M (2019) An aptamer based fluorometric microcystin-LR assay using DNA strand-based competitive displacement. Microchim. Acta 186(7):435. https://doi.org/10.1007/s00604-019-3504-8
Vasas G, Gáspár A, Páger C, Surányi G, Máthé C, Hamvas MM, Borbely G (2004) Analysis of cyanobacterial toxins (anatoxin-a, cylindrospermopsin, microcystin-LR) by capillary electrophoresis. Electrophoresis 25(1):108–115. https://doi.org/10.1002/elps.200305641
Sassolas A, Catanante G, Fournier D, Marty JL (2011) Development of a colorimetric inhibition assay for microcystin-LR detection: comparison of the sensitivity of different protein phosphatases. Talanta 85(5):2498–2503. https://doi.org/10.1016/j.talanta.2011.07.101
Chen J, Gao P, Wang H, Han L, Zhang Y, Wang P, Jia N (2018) A PPy/Cu2O molecularly imprinted composite film-based visible light-responsive photoelectrochemical sensor for microcystin-LR. J Mater Chem 6(15):3937–3944. https://doi.org/10.1039/C7TC05743A
Li L, Chen Y, Zhu J-J (2017) Recent advances in electrochemiluminescence analysis. Anal Chem 89(1):358–371. https://doi.org/10.1021/acs.analchem.6b04675
Hao N, Lu J, Dai Z, Qian J, Zhang J, Guo Y, Wang K (2019) Analysis of aqueous systems using all-inorganic perovskite CsPbBr3 quantum dots with stable electrochemiluminescence performance using a closed bipolar electrode. Electrochem Commun 108:106559. https://doi.org/10.1016/j.elecom.2019.106559
Hao N, Zhang X, Zhou Z, Hua R, Zhang Y, Liu Q, Qian J, Li H, Wang K (2017) AgBr nanoparticles/3D nitrogen-doped graphene hydrogel for fabricating all-solid-state luminol-electrochemiluminescence Escherichia coli aptasensors. Biosens Bioelectron 97:377–383. https://doi.org/10.1016/j.bios.2017.06.025
Huan J, Liu Q, Fei A, Qian J, Dong X, Qiu B, Mao H, Wang K (2015) Amplified solid-state electrochemiluminescence detection of cholesterol in near-infrared range based on CdTe quantum dots decorated multiwalled carbon nanotubes @reduced graphene oxide nanoribbons. Biosens Bioelectron 73:221–227. https://doi.org/10.1016/j.bios.2015.06.004
Su Q, Feng W, Yang D, Li F (2017) Resonance energy transfer in upconversion nanoplatforms for selective biodetection. Acc Chem Res 50(1):32–40. https://doi.org/10.1021/acs.accounts.6b00382
Wang J, Shan Y, Zhao W-W, Xu J-J, Chen H-Y (2011) Gold nanoparticle enhanced electrochemiluminescence of CdS thin films for ultrasensitive thrombin detection. Anal Chem 83(11):4004–4011. https://doi.org/10.1021/ac200616g
Ma H, Li X, Yan T, Li Y, Liu H, Zhang Y, Wu D, Du B, Wei Q (2016) Sensitive insulin detection based on electrogenerated chemiluminescence resonance energy transfer between Ru(bpy)32+ and au nanoparticle-doped β-cyclodextrin-Pb (II) metal–organic framework. ACS Appl Mater Interfaces 8(16):10121–10127. https://doi.org/10.1021/acsami.5b11991
Fu X-L, Hou F, Liu F-R, Ren S-W, Cao J-T, Liu Y-M (2019) Electrochemiluminescence energy resonance transfer in 2D/2D heterostructured g-C3N4/MnO2 for glutathione detection. Biosens Bioelectron 129:72–78. https://doi.org/10.1016/j.bios.2019.01.010
Dennis AM, Bao G (2008) Quantum dot−fluorescent protein pairs as novel fluorescence resonance energy transfer probes. Nano Lett 8(5):1439–1445. https://doi.org/10.1021/nl080358+
Sapsford KE, Granek J, Deschamps JR, Boeneman K, Blanco-Canosa JB, Dawson PE, Susumu K, Stewart MH, Medintz IL (2011) Monitoring botulinum neurotoxin a activity with peptide-functionalized quantum dot resonance energy transfer sensors. ACS Nano 5(4):2687–2699. https://doi.org/10.1021/nn102997b
Zhu W, Wang C, Li X, Khan MS, Sun X, Ma H, Fan D, Wei Q (2017) Zinc-doping enhanced cadmium sulfide electrochemiluminescence behavior based on au-cu alloy nanocrystals quenching for insulin detection. Biosens Bioelectron 97:115–121. https://doi.org/10.1016/j.bios.2017.05.046
Liu Q, Huan J, Dong X, Qian J, Hao N, You T, Mao H, Wang K (2016) Resonance energy transfer from CdTe quantum dots to gold nanorods using MWCNTs/rGO nanoribbons as efficient signal amplifier for fabricating visible-light-driven “on-off-on” photoelectrochemical acetamiprid aptasensor. Sensors Actuators B Chem 235:647–654. https://doi.org/10.1016/j.snb.2016.05.154
Song X, Li X, Wei D, Feng R, Yan T, Wang Y, Ren X, Du B, Ma H, Wei Q (2019) CuS as co-reaction accelerator in PTCA-K2S2O8 system for enhancing electrochemiluminescence behavior of PTCA and its application in detection of amyloid-β protein. Biosens Bioelectron 126:222–229. https://doi.org/10.1016/j.bios.2018.10.068
Cui Y, Li B, He H, Zhou W, Chen B, Qian G (2016) Metal–organic frameworks as platforms for functional materials. Acc Chem Res 49(3):483–493. https://doi.org/10.1021/acs.accounts.5b00530
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822. https://doi.org/10.1038/346818a0
Yang M, Liu G, Mehedi HM, Ouyang Q, Chen Q (2017) A universal SERS aptasensor based on DTNB labeled GNTs/Ag core-shell nanotriangle and CS-Fe3O4 magnetic-bead trace detection of aflatoxin B1. Anal Chim Acta 986:122–130. https://doi.org/10.1016/j.aca.2017.07.016
Lei Y-M, Wen R-X, Zhou J, Chai Y-Q, Yuan R, Zhuo Y (2018) Silver ions as novel Coreaction accelerator for remarkably enhanced electrochemiluminescence in a PTCA–S2O82− system and its application in an ultrasensitive assay for mercury ions. Anal Chem 90(11):6851–6858. https://doi.org/10.1021/acs.analchem.8b01018
Kim S-N, Kim J, Kim H-Y, Cho H-Y, Ahn W-S (2013) Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal Today 204:85–93. https://doi.org/10.1016/j.cattod.2012.08.014
Li J, Shan X, Jiang D, Chen Z (2020) An ultrasensitive electrochemiluminescence aptasensor for the detection of diethylstilbestrol based on the enhancing mechanism of the metal–organic framework NH2-MIL-125(Ti) in a 3,4,9,10-perylenetetracarboxylic acid/K2S2O8 system. Analyst 145(9):3306–3312. https://doi.org/10.1039/D0AN00212G
Zhu D, Yan Y, Lei P, Shen B, Cheng W, Ju H, Ding S (2014) A novel electrochemical sensing strategy for rapid and ultrasensitive detection of Salmonella by rolling circle amplification and DNA–AuNPs probe. Anal Chim Acta 846:44–50. https://doi.org/10.1016/j.aca.2014.07.024
Wen G, Guo Z (2018) Facile modification of NH2-MIL-125(Ti) to enhance water stability for efficient adsorptive removal of crystal violet from aqueous solution. Colloids Surf A Physicochem Eng Asp 541:58–67. https://doi.org/10.1016/j.colsurfa.2018.01.011
Fu X, Yang Y, Wang N, Chen S (2017) The electrochemiluminescence resonance energy transfer between Fe-MIL-88 metal–organic framework and 3,4,9,10-perylenetetracar-boxylic acid for dopamine sensing. Sensors Actuators B Chem 250:584–590. https://doi.org/10.1016/j.snb.2017.04.054
Zhu D, Yan Y, Lei P, Shen B, Cheng W, Ju H, Ding S (2014) A novel electrochemical sensing strategy for rapid and ultrasensitive detection of Salmonella by rolling circle amplification and DNA-AuNPs probe. Anal Chim Acta 846:44–50. https://doi.org/10.1016/j.aca.2014.07.024
Zhao G, Wang Y, Li X, Dong X, Wang H, Du B, Cao W, Wei Q (2018) Quenching electrochemiluminescence Immunosensor based on resonance energy transfer between ruthenium (II) complex incorporated in the UiO-67 metal-organic framework and gold nanoparticles for insulin detection. ACS Appl Mater Interfaces 10(27):22932–22938. https://doi.org/10.1021/acsami.8b04786
Liu L, Shan D, Zhou X, Shi H, Song B, Falke F, Leinse A, Heideman R (2018) TriPleX™ waveguide-based fluorescence biosensor for multichannel environmental contaminants detection. Biosens Bioelectron 106:117–121. https://doi.org/10.1016/j.bios.2018.01.066
Ríos V, Moreno I, Prieto AI, Puerto M, Gutiérrez-Praena D, Soria-Díaz ME, Cameán AM (2013) Analysis of MC-LR and MC-RR in tissue from freshwater fish (Tinca tinca) and crayfish (Procambarus clarkii) in tench ponds (Cáceres, Spain) by liquid chromatography–mass spectrometry (LC–MS). Food Chem Toxicol 57:170–178. https://doi.org/10.1016/j.fct.2013.03.025
Liu X, Tang Y, Liu P, Yang L, Li L, Zhang Q, Zhou Y, Khan MZH (2019) A highly sensitive electrochemical aptasensor for detection of microcystin-LR based on a dual signal amplification strategy. Analyst 144(5):1671–1678. https://doi.org/10.1039/C8AN01971A
Lin Z, Huang H, Xu Y, Gao X, Qiu B, Chen X, Chen G (2013) Determination of microcystin-LR in water by a label-free aptamer based electrochemical impedance biosensor. Talanta 103:371–374. https://doi.org/10.1016/j.talanta.2012.10.081
Ruiyi L, Qianfang X, Zaijun L, Xiulan S, Junkang L (2013) Electrochemical immunosensor for ultrasensitive detection of microcystin-LR based on graphene–gold nanocomposite/functional conducting polymer/gold nanoparticle/ionic liquid composite film with electrodeposition. Biosens Bioelectron 44:235–240. https://doi.org/10.1016/j.bios.2013.01.007
Funding
This work was financially supported by the Natural Science Foundation of Jiangsu Province (BK20190928), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJB150001, 19KJB150003), the National Natural Science Foundation of China (51874050, Nos. 51874050 and 21904014) and the Foundation of Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology (BM2012110). This work was also supported in part by Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX20_0946).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3766 kb)
Rights and permissions
About this article
Cite this article
Li, J., Jiang, D., Shan, X. et al. An “off-on” electrochemiluminescence aptasensor for microcystin-LR assay based on the resonance energy transfer from PTCA/NH2-MIL-125(Ti) to gold nanoparticles. Microchim Acta 187, 474 (2020). https://doi.org/10.1007/s00604-020-04453-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04453-x