Abstract
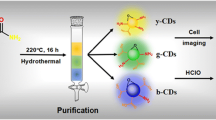
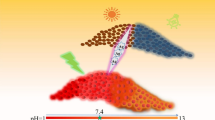
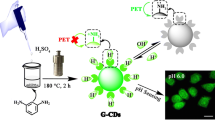
Dual-emission carbon dots were synthesized by one-pot hydrothermal pyrolysis of citric acid and polyethyleneimine in the presence of rhodamine B at 160 °C for 5 h. The carbon dots have an average diameter of 2.51 nm with rhodamine moiety on their surface. Two emission bands centered at 447 and 581 nm are exhibited in their fluorescence spectra excited at 360 nm, and the former is sensitive while the latter is insensitive to Hg2+ and pH. Glutathione (GSH) can recover the fluorescence quenched by Hg2+. Therefore, the dual-emission carbon dots were developed as a fluorescent ratiometric probe employing the ratio of the two intensities at 447 and 581 nm (RI447/I581) as the signal for the determinations of pH, Hg2+, and GSH. In the range of 5.0–10.0, a good linear relationship between RI447/I581 and pH was built with a regression equation of RI447/I581 = 11.95–0.56 pH (R2 = 0.998). In the range from 0.0 to 8.0 μM, an excellent linear relationship between RI447/I581 and the concentration of Hg2+ was obtained with a calibration equation of RI447/I581 = 6.2317–0.4458c (R2 = 0.995) and a limit of detection (LOD) of 0.24 μM. In the range from 1.0 to 10.0 μM, a linear equation, RI447/I581 = 1.9133–0.4157c (R2 = 0.995), was calibrated between RI447/I581 and the concentration of glutathione with a LOD of 0.27 μM. The recoveries for the determinations of Hg2+ and GSH in real samples were in the ranges of 94.6 to 103.8% and 94.3 to 104.2%, respectively.

Dual-emission carbon dots achieved by luminescence center modulation within one-pot synthesis for a fluorescent ratiometric probe of pH, Hg2+, and glutathione






Similar content being viewed by others
References
Pan X, Zhang Y, Sun X, Pan W, Yu G, Si S, Wang J (2018) The effect of carbon chain length of starting materials on the formation of carbon dots and their optical properties. Mater Res Express 5(4):045603. https://doi.org/10.1088/2053-1591/aaba5e
Tian Z, Zhang X, Li D, Zhou D, Jing P, Shen D, Qu S, Zboril R, Rogach AL (2017) Full-color inorganic carbon dot phosphors for white-light-emitting diodes. Adv Opt Mater 5(19):1700416. https://doi.org/10.1002/adom.201700416
Pan X, Zhang Y, Sun X, Pan W, Wang J (2018) A green emissive carbon-dot-based sensor with diverse responsive manners for multi-mode sensing. Analyst 143(23):5812–5821. https://doi.org/10.1039/c8an01552j
Li H, Shao F-Q, Huang H, Feng J-J, Wang A-J (2016) Eco-friendly and rapid microwave synthesis of green fluorescent graphitic carbon nitride quantum dots for vitro bioimaging. Sens and Actuators B: Chem 226:506–511. https://doi.org/10.1016/j.snb.2015.12.018
Pan X, Zhang Y, Sun X, Pan W, Yu G, Zhao Q, Wang J (2018) Carbon dots originated from methyl red with molecular state and surface state controlled emissions for sensing and imaging. J Lumin 204:303–311. https://doi.org/10.1016/j.jlumin.2018.08.007
Jian X, Li J-g, Yang H-m, Cao L-l, Zhang E-h, Liang Z-h (2017) Carbon quantum dots reinforced polypyrrole nanowire via electrostatic self-assembly strategy for high-performance supercapacitors. Carbon 114:533–543. https://doi.org/10.1016/j.carbon.2016.12.033
Liu W, Li C, Sun X, Pan W, Wang J (2017) Carbon-dot-based ratiometric fluorescent pH sensor for the detections of very weak acids assisted by auxiliary reagents that contribute to the release of protons. Sens and Actuators B: Chem 244:441–449. https://doi.org/10.1016/j.snb.2017.01.009
Gu D, Shang S, Yu Q, Shen J (2016) Green synthesis of nitrogen-doped carbon dots from lotus root for Hg (II) ions detection and cell imaging. Appl Surf Sci 390:38–42. https://doi.org/10.1016/j.apsusc.2016.08.012
Zhou J, Zhou H, Tang J, Deng S, Yan F, Li W, Qu M (2016) Carbon dots doped with heteroatoms for fluorescent bioimaging: a review. Microchim Acta 184(2):343–368. https://doi.org/10.1007/s00604-016-2043-9
Zuo P, Lu X, Sun Z, Guo Y, He H (2015) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183(2):519–542. https://doi.org/10.1007/s00604-015-1705-3
Lu L, Feng C, Xu J, Wang F, Yu H, Xu Z, Zhang W (2017) Hydrophobic-carbon-dot-based dual-emission micelle for ratiometric fluorescence biosensing and imaging of Cu(2+) in liver cells. Biosens Bioelectron 92:101–108. https://doi.org/10.1016/j.bios.2017.01.066
Gao X, Ding C, Zhu A, Tian Y (2014) Carbon-dot-based ratiometric fluorescent probe for imaging and biosensing of superoxide anion in live cells. Anal Chem 86(14):7071–7078. https://doi.org/10.1021/ac501499y
Xiang GQ, Ren Y, Xia Y, Mao W, Fan C, Guo SY, Wang PP, Yang DH, He L, Jiang X (2017) Carbon-dot-based dual-emission silica nanoparticles as a ratiometric fluorescent probe for bisphenol A. Spectrochim Acta A Mol Biomol Spectrosc 177:153–157. https://doi.org/10.1016/j.saa.2017.01.049
Yuan YH, Liu ZX, Li RS, Zou HY, Lin M, Liu H, Huang CZ (2016) Synthesis of nitrogen-doping carbon dots with different photoluminescence properties by controlling the surface states. Nanoscale 8(12):6770–6776. https://doi.org/10.1039/c6nr00402d
Sarkar S, Banerjee D, Ghorai UK, Das NS, Chattopadhyay KK (2016) Size dependent photoluminescence property of hydrothermally synthesized crystalline carbon quantum dots. J Lumin 178:314–323. https://doi.org/10.1016/j.jlumin.2016.05.033
Zhu S, Zhao X, Song Y, Lu S, Yang B (2016) Beyond bottom-up carbon nanodots: citric-acid derived organic molecules. Nano Today 11(2):128–132. https://doi.org/10.1016/j.nantod.2015.09.002
Shi L, Yang JH, Zeng HB, Chen YM, Yang SC, Wu C, Zeng H, Yoshihito O, Zhang Q (2016) Carbon dots with high fluorescence quantum yield: the fluorescence originates from organic fluorophores. Nanoscale 8(30):14374–14378. https://doi.org/10.1039/c6nr00451b
Yan F, Jiang Y, Sun X, Bai Z, Zhang Y, Zhou X (2018) Surface modification and chemical functionalization of carbon dots: a review. Mikrochim Acta 185(9):424. https://doi.org/10.1007/s00604-018-2953-9
Zou W-S, Zhao Q-C, Zhang J, Chen X-M, Wang X-F, Zhao L, Chen S-H, Wang Y-Q (2017) Enhanced photoresponsive polyethyleneimine/citric acid co-carbonized dots for facile and selective sensing and intracellular imaging of cobalt ions at physiologic pH. Anal Chim Acta 970:64–72. https://doi.org/10.1016/j.aca.2017.03.018
Zou W-S, Zhao Q-C, Kong W-L, Wang X-F, Chen X-M, Zhang J, Wang Y-Q (2018) Multi-level fluorescent logic gate based on polyamine coated carbon dots capable of responding to four stimuli. Chem Eng J 337:471–479. https://doi.org/10.1016/j.cej.2017.12.123
Dong Y, Chen Y, You X, Lin W, Lu CH, Yang HH, Chi Y (2017) High photoluminescent carbon based dots with tunable emission color from orange to green. Nanoscale 9(3):1028–1032. https://doi.org/10.1039/c6nr08444c
Jin L, Shi L, Shi W, Meng Z, Shang L, Shen Y (2019) Fluorescence lifetime-based pH sensing by platinum nanoclusters. Analyst 144(11):3533–3538. https://doi.org/10.1039/c9an00061e
Kong L, Chu X, Wang C, Zhou H, Wu Y, Liu W (2018) D-Penicillamine-coated Cu/Ag alloy nanocluster superstructures: aggregation-induced emission and tunable photoluminescence from red to orange. Nanoscale 10(4):1631–1640. https://doi.org/10.1039/c7nr08434j
Baffou G, Rigneault H, Marguet D, Jullien L (2014) A critique of methods for temperature imaging in single cells. Nat Methods 11(9):899–901. https://doi.org/10.1038/nmeth.3073
Bomm J, Günter C, Stumpe J (2011) Synthesis and optical characterization of thermosensitive, luminescent gold nanodots. J Phys Chem C 116(1):81–85. https://doi.org/10.1021/jp206260r
Yu J, Song N, Zhang Y-K, Zhong S-X, Wang A-J, Chen J (2015) Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sens and Actuators B: Chem 214:29–35. https://doi.org/10.1016/j.snb.2015.03.006
Yang S, Sun J, Li X, Zhou W, Wang Z, He P, Ding G, Xie X, Kang Z, Jiang M (2014) Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J Mater Chem A 2(23):8660. https://doi.org/10.1039/c4ta00860j
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184(7):1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Chen X, Zhou Y, Peng X, Yoon J (2010) Fluorescent and colorimetric probes for detection of thiols. Chem Soc Rev 39(6):2120–2135. https://doi.org/10.1039/b925092a
Wang H, Lu Q, Li M, Li H, Liu Y, Li H, Zhang Y, Yao S (2018) Electrochemically prepared oxygen and sulfur co-doped graphitic carbon nitride quantum dots for fluorescence determination of copper and silver ions and biothiols. Anal Chim Acta 1027:121–129. https://doi.org/10.1016/j.aca.2018.03.063
Yan F, Shi D, Zheng T, Yun K, Zhou X, Chen L (2016) Carbon dots as nanosensor for sensitive and selective detection of Hg2+ and l-cysteine by means of fluorescence “Off–On” switching. Sens Actuators B Chem 224:926–935. https://doi.org/10.1016/j.snb.2015.09.057
Xu S, Liu Y, Yang H, Zhao K, Li J, Deng A (2017) Fluorescent nitrogen and sulfur co-doped carbon dots from casein and their applications for sensitive detection of Hg(2+) and biothiols and cellular imaging. Anal Chim Acta 964:150–160. https://doi.org/10.1016/j.aca.2017.01.037
Guo Y, Yang L, Li W, Wang X, Shang Y, Li B (2016) Carbon dots doped with nitrogen and sulfur and loaded with copper (II) as a “turn-on” fluorescent probe for cystein, glutathione and homocysteine. Microchim Acta 183(4):1409–1416. https://doi.org/10.1007/s00604-016-1779-6
Ratola N, Santos L, Herbert P, Alves A (2006) Uncertainty associated to the analysis of organochlorine pesticides in water by solid-phase microextraction/gas chromatography-electron capture detection--evaluation using two different approaches. Anal Chim Acta 573-574:202–208. https://doi.org/10.1016/j.aca.2006.03.065
Chen Z, Lin Y, Zhao C, Ren J, Qu X (2012) Silver metallization engineered conformational switch of G-quadruplex for fluorescence turn-on detection of biothiols. Chem Commun (Camb) 48(93):11428–11430. https://doi.org/10.1039/c2cc36690h
Funding
This work was financially supported by the National Key R&D Program of China (2017YFE0105200), Key Research and Development Project of Shandong Province (No. 2018GNC110006), and National Natural Science Foundation of China (No. 31572181).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All procedures performed in this work involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoyu Wang, co-first author.
Electronic supplementary material
ESM 1
(DOCX 651 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Wang, X., Pan, X. et al. Dual-emission carbon dots achieved by luminescence center modulation within one-pot synthesis for a fluorescent ratiometric probe of pH, Hg2+, and glutathione. Microchim Acta 187, 330 (2020). https://doi.org/10.1007/s00604-020-04311-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04311-w