Abstract
The authors describe a chemiluminescence (CL)-based assay for the determination of bromate. The method is based on the use of a solution of carbon quantum dots (CQDs) and sulfite. Strong CL (peak at 490 nm) is observed when bromate is injected into the solution. The CL increases linearly in the 0.3 to 10 μmol L−1 bromate concentration range, giving a 0.1 μmol L−1 limit of detection (at an S/N ratio of 3). A possible CL mechanism is suggested that involves a redox reaction between the CQDs, bromate and sulfite in the acidic medium. This leads to the formation of hole-injected and electron-injected CQDs. Radiative recombination of oxidant-injected holes and electrons in the CQDs accounts for the occurrence of CL. This mechanism contradicts the previous assumption that the transfer of energy occurs from SO2* to the CQDs. Although nitrite may interfere in the determination of bromate, its effect can be eliminated by adding sulfamic acid. The assay is sensitive and represents a new tool for the determination of bromate, which is a carcinogen.

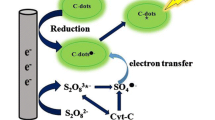
Under acidic condition, carbon quantum dots (CQDs) can react with sulfite and bromate transforming to hole-injected CQDs (CQDs•–) and electron-injected CQDs (CQDs•+), respectively. Thereafter, strong chemiluminescence (490 nm) aroused from the radiative electron-hole annihilation between CQDs•– and CQDs•+.



Similar content being viewed by others
References
IARC (1987) Monographs on the evaluation of the carcinogenic risk of chemicals to humans, overall evaluation of the carcinogenicity: an updating of iarc monographs, vol 1–42 Suppl 7
The council of the European Union (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Official Journal L 330:0032–0054
Health Canada (2017) Guidelines for Canadian drinking water quality—summary table. Water and air quality bureau, healthy environments and consumer safety branch, health Canada, Ottawa
National Standards of P. R. China (2006) Hygienic standard for drinking water (GB 5749–2006)
National Standards of P. R. China (2008) Drinking natural mineral water (GB 8537–2008)
National Standards of P. R. China (2014). National standard for food safety- Packaged drinking water (GB 19298–2014)
WHO (2011) Guideline for drinking-water quality, 4th edn
Michalski R, Lyko A (2013) Bromate determination: state of the art. Crit Rev Anal Chem 43:100–122. https://doi.org/10.1080/10408347.2012.747792
Ke-Tai W, Hui-Tao L, Jian H, Xing-Guo C, Zhi-De H (2000) Determination of bromate in bread additives and flours by flow injection analysis. Food Chem 70:509–514
van Staden JF, Mulaudzi LV, Stefan RI (2004) Spectrophotometric determination of bromate by sequential injection analysis. Talanta 64:1196–1202. https://doi.org/10.1016/j.talanta.2004.04.031
Al Okab RA (2013) Cisapride a green analytical reagent for rapid and sensitive determination of bromate in drinking water, bread and flour additives by oxidative coupling spectrophotometric methods. Spectrochim Acta A 103:333–337. https://doi.org/10.1016/j.saa.2012.10.071
Farmany A, Mortazavi SS, Hashemi E, Sahraei R (2014) A new catalytic oxidation method for sensitive quantification of bromate in flours and bottled water using AgNPs. Environ Monit Assess 186:1371–1375. https://doi.org/10.1007/s10661-013-3459-x
Rocha DL, Machado MC, Melchert WR (2014) A sensitive flow-based procedure for spectrophotometric speciation analysis of inorganic bromine in waters. Talanta 129:93–99. https://doi.org/10.1016/j.talanta.2014.05.013
Lim HH, Shin HS (2012) Sensitive and robotic determination of bromate in sea water and drinking deep-sea water by headspace solid-phase micro extraction and gas chromatography-mass spectrometry. Anal Chim Acta 741:32–37. https://doi.org/10.1016/j.aca.2012.06.046
Shin HS (2012) Sensitive determination of bromate in ozonated and chlorinated water, and sea water by gas chromatography-mass spectrometry after derivatization. J Chromatogr A 1223:136–141. https://doi.org/10.1016/j.chroma.2011.12.059
Yokota A, Kubota H, Komiya S, Sato K, Akiyama H, Koshiishi I (2012) Sensitive and simple determination of bromate in foods disinfected with hypochlorite reagents using high performance liquid chromatography with post-column derivatization. J Chromatogr A 1262:219–222. https://doi.org/10.1016/j.chroma.2012.09.014
Fotsing M, Barbeau B, Prevost M (2011) Low-level bromate analysis in drinking water by ion chromatography with optimized suppressed conductivity cell current followed by a post-column reaction and UV/Vis detection. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:420–425. https://doi.org/10.1080/10934529.2011.542401
Cordeiro F, Robouch P, de la Calle MB, Emteborg H, Charoud-Got J, Schmitz F (2011) Determination of dissolved bromate in drinking water by ion chromatography and post column reaction: interlaboratory study. J AOAC Int 94:1592–1600. https://doi.org/10.5740/jaoacint.10-404
Wang N, He S, Zhu Y (2012) Low-level bromate analysis by ion chromatography on a polymethacrylate-based monolithic column followed by a post-column reaction. Eur Food Res Technol 235:685–692. https://doi.org/10.1007/s00217-012-1800-1
Akiyama T, Yamanaka M, Date Y (2002) Specific determination of bromate in bread by ion chromatography with ICP-MS. J Food Hyg Soc Jpn 43:348–351
Silva J, Dias J, Magalhães J (2001) Factorial analysis of a chemiluminescence system for bromate detection in water. Anal Chim Acta 450:175–184
Yan Z, Zhang Z, Yu Y, Liu Z, Chen J (2016) Chemiluminescence determination of potassium bromate in flour based on flow injection analysis. Food Chem 190:20–24. https://doi.org/10.1016/j.foodchem.2015.05.076
Chen H, Lin L, Li H, Lin J-M (2014) Quantum dots-enhanced chemiluminescence: mechanism and application. Coord Chem Rev 263–264:15
Shah SNA, Lin J-M (2017) Recent advances in chemiluminescence based on carbonaceous dots. Adv Colloid Interf Sci 241:24–36. https://doi.org/10.1016/j.cis.2017.01.003
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184:1899–1914. https://doi.org/10.1007/s00604-017-2318-9
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381. https://doi.org/10.1039/c4cs00269e
Yang N, Swain GM, Jiang X (2016) Nanocarbon electrochemistry and electroanalysis: current status and future perspectives. Electroanalysis 28:27–34. https://doi.org/10.1002/elan.201500577
Wang R, K-Q L, Tang Z-R, Y-J X (2017) Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis. J Mater Chem A 5:3717–3734. https://doi.org/10.1039/c6ta08660h
Imani-Nabiyyi A, Sorouraddin MH, Amjadi M, Naseri A (2014) Luminol/CdTe quantum dots/sodium periodate system in conjunction with response-surface methodology for chemiluminometric determination of some tetracyclines. J Lumin 151:57–65. https://doi.org/10.1016/j.jlumin.2014.01.075
Khataee A, Hasanzadeh A, Iranifam M, Joo SW (2015) A novel flow-injection chemiluminescence method for determination of baclofen using l-cysteine capped CdS quantum dots. Sensors Actuators B Chem 215:11. https://doi.org/10.1016/j.snb.2015.03.066
Dong YP, Gao TT, Zhou Y, Zhu JJ (2014) Electrogenerated chemiluminescence resonance energy transfer between luminol and CdSe@ZnS quantum dots and its sensing application in the determination of thrombin. Anal Chem 86:11373–11379. https://doi.org/10.1021/ac5033319
Lin Z, Xue W, Chen H, Lin JM (2012) Classical oxidant induced chemiluminescence of fluorescent carbon dots. Chem Commun 48:1051–1053. https://doi.org/10.1039/c1cc15290d
Ding Z, Quinn BM, Haram SK, Pell LE, Korgel BA, Bard AJ (2002) Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science 296:1293–1297. https://doi.org/10.1126/science.1069336
Zheng L, Chi Y, Dong Y, Lin J, Wang B (2009) Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphite. J Am Chem Soc 131:4564–4565. https://doi.org/10.1021/ja809073f
Lin Z, Dou X, Li H, Ma Y, Lin J-M (2015) Nitrite sensing based on the carbon dots-enhanced chemiluminescence from peroxynitrous acid and carbonate. Talanta 132:457–462. https://doi.org/10.1016/j.talanta.2014.09.046
Vilian ATE, Chen S-M, Kwak CH, Hwang S-K, Huh YS, Han Y-K (2016) Immobilization of hemoglobin on functionalized multi-walled carbon nanotubes-poly-l-histidine-zinc oxide nanocomposites toward the detection of bromate and H2O2. Sensors Actuators B Chem 224:607–617. https://doi.org/10.1016/j.snb.2015.10.099
Majidi MR, Ghaderi S, Asadpour-Zeynali K, Dastangoo H (2015) Electrochemical determination of bromate in different types of flour and bread by a sensitive Amperometric sensor based on palladium nanoparticles/graphene oxide Nanosheets. Food Anal Methods 8:2011–2019. https://doi.org/10.1007/s12161-014-0065-7
Saraji M, Khaje N, Ghani M (2014) Cetyltrimethylammonium-coated magnetic nanoparticles for the extraction of bromate, followed by its spectrophotometric determination. Microchim Acta 181:925–933. https://doi.org/10.1007/s00604-014-1188-7
Palanisamy S, Wang Y-T, Chen S-M, Thirumalraj B, Lou B-S (2016) Direct electrochemistry of immobilized hemoglobin and sensing of bromate at a glassy carbon electrode modified with graphene and β-cyclodextrin. Microchim Acta 183:1953–1961. https://doi.org/10.1007/s00604-016-1811-x
Menendez-Miranda M, Fernandez-Argüelles MT, Costa-Fernandez JM, Pereiro R, Sanz-Medel A (2013) Room temperature phosphorimetric determination of bromate in flour based on energy transfer. Talanta 116:231–236. https://doi.org/10.1016/j.talanta.2013.05.019
Acknowledgements
The authors would like to acknowledge financial support from the Natural Science Foundation of China (Nos. 81760601, 81260435 and 21265013), the Natural Science Foundation of Jiangxi Province (No. 20471BAB15050), the Social Development Project of Yunnan Province (No. 2013RA012) and the Innovation Fund Designated for Graduate Students of Nanchang University (No. cx2016372).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Liping Li and Xiaojing Lai are co-first authors.
Electronic supplementary material
ESM 1
(DOCX 174 kb)
Rights and permissions
About this article
Cite this article
Li, L., Lai, X., Xu, X. et al. Determination of bromate via the chemiluminescence generated in the sulfite and carbon quantum dot system. Microchim Acta 185, 136 (2018). https://doi.org/10.1007/s00604-017-2653-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2653-x