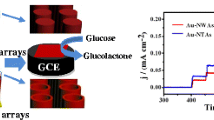
Abstract
The authors describe a template-free method for the electrodeposition of ultra-long copper nanowires on titanium foils. Scanning electron microscopy shows that the nanowires are around 50 nm in diameter and 30 μm in length. The titanium foils enable nonenzymatic sensing of glucose in 0.1 M NaOH solution because the nanowire-modified electrodes exhibit excellent electrocatalytic activity towards glucose oxidation at a typical working voltage of 0.7 V (vs. Ag/AgCl). Figures of merit include (a) a sensitivity of 4985 μA·mM−1·cm−2, (b) a linear response extending from 1 μM to 6.0 mM of glucose, (c) good reusability (a 2.5% relative standard deviation of one electrode in five detections), and (d) an excellent reproducibility (a 3.3% RSD of five electrodes to one sample).

Copper nanowires with high length-diameter ratio were electrodeposited on titanium foils as nonenzymatic sensors of glucose. Benefitting from the good conductivity, abundant active sites and facilitated mass transport, the nanowire-modified electrodes exhibit excellent electrocatalytic activity towards glucose oxidation.




Similar content being viewed by others
References
Chan TC, Lin YM, Tsai HW, Wang ZMM, Liao CN, Chueh YL (2014) Growth of large-scale nanotwinned cu nanowire arrays from anodic aluminum oxide membrane by electrochemical deposition process: controllable nanotwin density and growth orientation with enhanced electrical endurance performance. Nano 6:7332–7338
Zhang L, Zhang JY, Yang CL, Zhao GY, Mu JS, Wang Y (2015) Freestanding cu nanowire arrays on Ti/Cr/Si substrate as tough nonenzymatic glucose sensors. RSC Adv 5:82998–83003
Liu XM, Zhou YC (2005) Electrochemical synthesis and room temperature oxidation behavior of cu nanowires. J Mater Res 20:2371–2378
Zhao YX, Zhang Y, Li YP, Yan ZF (2012) A flexible chemical vapor deposition method to synthesize copper@carbon core–shell structured nanowires and the study of their structural electrical properties. New J Chem 36:1161–1169
Liu ZW, Bando YS (2003) A novel method for preparing copper nanorods and nanowires. Adv Mater 15:303–304
Meng F, Jin S (2012) The solution growth of copper nanowires and nanotubes is driven by screw dislocations. Nano Lett 12:234–239
Xiang HF, Long YH, Yu XL, Zhang XL, Zhao N, Xu J (2011) A novel and facile method to prepare porous hollow CuO and cu nanofibers based on electrospinning. Cryst Eng Commun 13:4856–4860
Liu ZP, Yang Y, Liang JB, Hu ZK, Li S, Peng S, Qian YT (2003) Synthesis of copper nanowires via a complex-surfactant-assisted hydrothermal reduction process. J Phys Chem B 107:12658–12661
Gambirasia A, Cattarina S, Musiania M, Vázquez-Gómeza L, Verlatoa E (2011) Direct electrodeposition of metal nanowires on electrode surface. Electrochim Acta 56:8582–8588
Chen X, Wu G, Cai Z, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Wang G, He X, Wang L, Gu A, Huang Y, Fang B, Geng B, Zhang X (2013) Non-enzymatic electrochemical sensing of glucose. Microchim Acta 180:161–186
Çiftçi H, Alver E, Çelik F, Metin AÜ, Tamer U (2016) Non-enzymatic sensing of glucose using a glassy carbon electrode modified with gold nanoparticles coated with polyethyleneimine and 3-aminophenylboronic acid. Microchim Acta 183:1479–1486
Mei L, Zhang P, Chen J, Chen D, Quan Y, Gu N, Zhang G, Cui R (2016) Non-enzymatic sensing of glucose and hydrogen peroxide using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous copper, carbon black and nafion. Microchim Acta 183:1359–1365
Dong JP, Ren LX, Zhang Y, Cui XL, Hu PF, Xu JQ (2015) Direct electrodeposition of cable-like CuO@cu nanowires array for non-enzymatic sensing. Talanta 132:719–726
Wang GF, Wei Y, Zhang W, Zhang XJ, Fang B, Wang L (2010) Enzyme-free amperometric sensing of glucose using cu-CuO nanowire composites. Microchim Acta 168:87–92
Mei H, Wu W, Yu B, Li Y, Wu H, Wang S, Xia Q (2015) Non-enzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with carbon supported co@Pt core-shell nanoparticles. Microchim Acta 182:1869–1875
Zhao L, Wu G, Cai Z, Zhao T, Yao Q, Chen X (2015) Ultrasensitive non-enzymatic glucose sensing at near-neutral pH values via anodic stripping voltammetry using a glassy carbon electrode modified with Pt3Pd nanoparticles and reduced graphene oxide. Microchim Acta 182:2055–2060
Zhong A, Luo X, Chen L, Wei S, Liang Y, Li X (2015) Enzyme-free sensing of glucose on a copper electrode modified with nickel nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182:1197–1204
Gao ZG, Lin YP, He Y, Tang DP (2017) Enzyme-free amperometric glucose sensor using a glassy carbon electrode modified with poly (vinyl butyral) incorporating a hybrid nanostructure composed of molybdenum disulfide and copper sulfide. Microchim Acta 184:807–814
Zhang C, Ni H, Chen R, Zhan W, Zhang B, Lei R, Xiao T, Zha Y (2015) Enzyme-free glucose sensing based on Fe3O4 nanorod arrays. Microchim Acta 182:1811–1818
Fan ZJ, Liu B, Liu XH, Li ZP, Wang HG, Yang SR, Wang JQ (2013) A flexible and disposable hybrid electrode based on cu nanowires modified graphene transparent electrode for non-enzymatic glucose sensor. Electrochim Acta 109:602–608
Jiang D, Liu Q, Wang K, Qian J, Dong XY, Yang ZT, Du XJ, Qiu BJ (2014) Enhanced non-enzymatic glucose sensing based on copper nanoparticles decorated nitrogen-doped graphene. Biosens Bioelectron 54:273–278
Zhang YC, Su L, Manuzzi D, Monteros HVE, Jia WZ, Huo DQ, Hou CJ, Lei Y (2012) Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens Bioelectron 31:426–432
Chen DJ, Lu YH, Wang AJ, Feng JJ, Huo TT, Dong WJ (2012) Facile synthesis of ultra-long cu microdendrites for the electrochemical detection of glucose. J Solid State Electrochem 16:1313–1321
Huang JW, Dong ZP, Li YD, Li J, Wang J, Yang HD, Li SW, Guo SJ, Jin J, Li R (2013) High performance non-enzymatic glucose biosensor based on copper nanowires–carbon nanotubes hybrid for intracellular glucose study. Sensors Actuators B Chem 182:618–624
Li H, Li YJ, Sun LL, Zhao XL (2013) One-step, template-free electrochemical preparation of three-dimensional porous au nanowire network and its enhancedactivity toward methanol electrooxidation. Electrochim Acta 108:74–78
Jana NR, Gearheart L, Murphy CJ (2001) Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem Commun 2001:617–618
Perez-Juste J, Liz-Marzan LM, Carnie S, Chan DYC, Mulvaney P (2004) Electric-field-directed grown of gold nanorods in aqueous surfactant solutions. Adv Funct Mater 14:571–579
Lu CH, Qi LM, Yang JH, Zhang DY, Wu NZ, Ma JM (2004) Simple template-free solution route for the controlled synthesis of cu(OH)2 and CuO nanostructures. J Phys Chem B 108:17825–17831
Mao CJ, Pan HC, Wu XC, Zhu JJ, Chen HY (2006) Sonochemical route for self-assembled V2O5 bundles with spindle-like morphology and their novel application in serum albumin sensing. J Phys Chem B 110:14709–14713
Li J, Zhang JH, Qian YT (2008) Surfactant-assisted synthesis of bundle-like nanostructures with well-aligned Te nanorods. Solid State Sci 10:1549–1555
Farrell ST, Breslin CB (2004) Oxidation and photo-induced oxidation of glucose at a polyaniline film modified by copper particles. Electrochim Acta 49:4497–4503
Torto N, Ruzgas T, Gorton L (1999) Electrochemical oxidation of mono- and disaccharides at fresh as well as oxidized copper electrodes in alkaline media. J Electroanal Chem 464:252–258
Kang XH, Mai ZB, Zou XY, Cai PX, Mo JY (2007) A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal Biochem 363:143–150
Babu TGS, Ramachandran T, Nair B (2010) Single step modification of copper electrode for the highly sensitive and selective non-enzymatic determination of glucose. Microchim Acta 169:49–55
Acknowledgements
This work was supported by the National Science Foundation of China (NSFC) (NO. 21203044, 31470489) and Fundamental Research Funds for the Central Universities (HIT. IBRSEM. A. 201407).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 827 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Ding, Y., Li, R. et al. Electrodeposition of ultra-long copper nanowires on a titanium foil electrode for nonenzymatic voltammetric sensing of glucose. Microchim Acta 184, 2837–2843 (2017). https://doi.org/10.1007/s00604-017-2279-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2279-z