Abstract
The article describes a sensitive and rapid method for the colorimetric determination of Salmonella pullorum and Salmonella gallinarum (S. pullorum and S. gallinarum). Silica coated magnetic nanoparticles (MNP) were modified with antibodies against S. pullorum and S. gallinarum to act as the capture probes. Horseradish peroxidase (HRP) and antibodies against S. pullorum and S. gallinarum on silica nanoparticles (HRP-IgG-SiNP) were used as detection probes (secondary antibody). In the presence of S. pullorum and S. gallinarum, the target bacteria are captured by capture probes and detection probes to form sandwich structures. This sandwich complexes were then magnetically isolated and used to catalytically oxidize the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (TMB). The absorbance at 450 nm is proportional to the concentration of S. pullorum and S. gallinarum. Under the optimized conditions, the assay has a detection range that extends from from 8.4 × 103 to 8.4 × 107 CFU⋅mL−1, and the limit of detection is 1.7 × 103 CFU⋅mL−1. The approach is cost-effective and specific. Sample preconcentration is not required. In our perception, this immunomagnetic nanoparticle-based detection strategy holds great promise for on-site detection of a wide range of pathogens by using the respective antibodies.

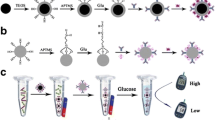
Schematic illustration of (a) the process for preparation of IgG-MNP, (b) the process for preparation of HRP-IgG-SiNP and (c) the colorimetric sandwich assay for detection of target bacteria S. pullorum and S.gallinarum by using HRP-loaded silica nanoparticle as the signal-transduction tag. (S. pullorum and S. gallinarum: Salmonella pullorum and Salmonella gallinarum; Ab: antibodies; HRP: Horseradish peroxidase; IgG-MNP: Antibodies against S. pullorum and S. gallinarum coated magnetic nanoparticles; HRP-IgG-SiNP: Horseradish peroxidase and antibodies against S. pullorum and S. gallinarum dual labled silica nanoparticles; TMB: 3,3′,5,5′-tetramethylbenzidine; APTES: 3-aminopropyltri-ethoxysilane).




Similar content being viewed by others
References
RAC PF, Ferreira JC, AMI K, ALdC D, Berchieri Junior A (2016) Antimicrobial susceptibility of salmonella Gallinarum and salmonella Pullorum isolated from ill poultry in Brazil. Ciência Rural 46:513–518. doi:10.1590/0103-8478cr20150398
Bagheryan Z, Raoof J-B, Golabi M, APF T, Beni V (2016) Diazonium-based impedimetric aptasensor for the rapid label-free detection of salmonella typhimurium in food sample. Biosens Bioelectron 80:566–573. doi:10.1016/j.bios.2016.02.024
de la Rica R, Stevens MM (2012) Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nano 7(12):821–824. doi:10.1038/nnano.2012.186
Kong D, Liu L, Xing C, Kuang H, Xu C (2015) Sensitive and highly specific detection of Cronobacter sakazakii based on monoclonal sandwich ELISA. Food Agric Immunol 26(4):566–576. doi:10.1080/09540105.2014.998634
Martin L, Chaabo A, Lasne F (2015) Detection of tetracosactide in plasma by enzyme-linked immunosorbent assay (ELISA). Drug Testing and Analysis 7(6):531–534. doi:10.1002/dta.1705
Lin J, Li M, Li Y, Chen Q (2015) A high gradient and strength bioseparator with nano-sized immunomagnetic particles for specific separation and efficient concentration of E. coli O157:H7. J Magn Magn Mater 378:206–213. doi:10.1016/j.jmmm.2014.11.039
Lai W, Wei Q, Zhuang J, Lu M, Tang D (2016) Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens Bioelectron 80:249–256. doi:10.1016/j.bios.2016.01.088
Chen L, Zhang Z, Zhang P, Zhang X, Fu A (2011) An ultra-sensitive chemiluminescence immunosensor of carcinoembryonic antigen using HRP-functionalized mesoporous silica nanoparticles as labels. Sensors Actuators B Chem 155(2):557–561. doi:10.1016/j.snb.2011.01.007
Ke R, Yang W, Xia X, Xu Y, Li Q (2010) Tandem conjugation of enzyme and antibody on silica nanoparticle for enzyme immunoassay. Anal Biochem 406(1):8–13. doi:10.1016/j.ab.2010.06.039
Zhang H, Lin L, Zeng X, Ruan Y, Wu Y, Lin M, He Y, Fu F (2016) Magnetic beads-based DNAzyme recognition and AuNPs-based enzymatic catalysis amplification for visual detection of trace uranyl ion in aqueous environment. Biosens Bioelectron 78:73–79. doi:10.1016/j.bios.2015.11.024
Bagwe RP, Yang C, Hilliard LR, Tan W (2004) Optimization of dye-doped silica nanoparticles prepared using a reverse Microemulsion method. Langmuir: the ACS journal of surfaces and colloids 20(19):8336–8342. doi:10.1021/la049137j
Chen L, Zhang Z, Zhang X, Fu A, Xue P, Yan R (2012) A novel chemiluminescence immunoassay of staphylococcal enterotoxin B using HRP-functionalised mesoporous silica nanoparticle as label. Food Chem 135(1):208–212. doi:10.1016/j.foodchem.2012.04.071
Wen C-Y, Hu J, Zhang Z-L, Tian Z-Q, Ou G-P, Liao Y-L, Li Y, Xie M, Sun Z-Y, Pang D-W (2013) One-step sensitive detection of salmonella typhimurium by coupling magnetic capture and fluorescence identification with functional Nanospheres. Anal Chem 85(2):1223–1230. doi:10.1021/ac303204q
Liu Y, Che Y, Li Y (2001) Rapid detection of salmonella typhimurium using immunomagnetic separation and immuno-optical sensing method. Sensors Actuators B Chem 72(3):214–218. doi:10.1016/S0925-4005(00)00663-8
Formisano N, Bhalla N, Heeran M, Reyes Martinez J, Sarkar A, Laabei M, Jolly P, Bowen CR, Taylor JT, Flitsch S, Estrela P (2016) Inexpensive and fast pathogenic bacteria screening using field-effect transistors. Biosens Bioelectron 85:103–109. doi:10.1016/j.bios.2016.04.063
Fei J, Dou W, Zhao G (2016) Amperometric immunoassay for the detection of salmonella pullorum using a screen - printed carbon electrode modified with gold nanoparticle-coated reduced graphene oxide and immunomagnetic beads. Microchim Acta 183(2):757–764. doi:10.1007/s00604-015-1721-3
Wan Y, Qi P, Zeng Y, Sun Y, Zhang D (2016) Invertase-mediated system for simple and rapid detection of pathogen. Sensors Actuators B Chem 233:454–458. doi:10.1016/j.snb.2016.04.098
Charlermroj R, Oplatowska M, Gajanandana O, Himananto O, Grant IR, Karoonuthaisiri N, Elliott CT (2013) Strategies to improve the surface plasmon resonance-based immmunodetection of bacterial cells. Microchim Acta 180(7):643–650. doi:10.1007/s00604-013-0975-x
Liu K, Yan X, Mao B, Wang S, Deng L (2016) Aptamer-based detection of salmonella enteritidis using double signal amplification by Klenow fragment and dual fluorescence. Microchim Acta 183(2):643–649. doi:10.1007/s00604-015-1692-4
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2016) Impedimetric salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 183(1):337–344. doi:10.1007/s00604-015-1649-7
Lei P, Tang H, Ding S, Ding X, Zhu D, Shen B, Cheng Q, Yan Y (2015) Determination of the invA gene of salmonella using surface plasmon resonance along with streptavidin aptamer amplification. Microchim Acta 182(1):289–296. doi:10.1007/s00604-014-1330-6
Acknowledgements
This work was financially supported by a grant from National Natural Science Foundation of Zhejiang Province (LY17C2000003), the Food and Engineering most important discipline of Zhejiang province (JYTSP20141062), Zhejiang public Innovation Platform Analysis and testing project (2015C37023), the Talent training provincial superior paper funded project (1110JY1412001P), Technological Innovation Projectof Zhejiang Gongshang University (CX201610024, CX201610019), Postgraduate Scientific and Technological Innovation Project of Zhejiang Gongshang University and plans of college students in Zhejiang province and technology innovation activities (acrobatic tender grass talent programme) project (1110JQ4212048G, 1110KZN0213112G).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
The original version of this article was revised: Table 1 contained error. Given in this article is the correct table.
An erratum to this article is available at http://dx.doi.org/10.1007/s00604-017-2274-4.
Electronic supplementary material
ESM 1
(DOCX 291 kb)
Rights and permissions
About this article
Cite this article
Zhu, H., Zhao, G., Wang, S.Q. et al. Photometric sandwich immunoassay for Salmonella pullorum and Salmonella gallinarum using horseradish peroxidase and magnetic silica nanoparticles. Microchim Acta 184, 1873–1880 (2017). https://doi.org/10.1007/s00604-017-2241-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2241-0