Abstract
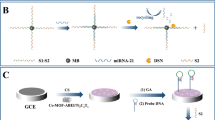
The authors describe a sensing interface that is capable of selectively adsorbing gold nanopartices (AuNPs). It was applied to electrochemiluminescent (ECL) detection of microRNA (miRNA). The AuNPs are used as signal transduction probes and luminol acts as the ECL reagent. The sensing interface was generated by sequential assembly of Nafion-carbon nanotubes (CNTs) and polyvinylpyrrolidone (PVP) at a glassy carbon electrode (GCE). If only ss-DNA probes and AuNPs are present, the interaction between them leads to the formation of ss-DNA-capped AuNPs. In this case, the capped-AuNP do not assemble at the interface of the modified electrode. As a result, the ECL is weak due to the poor electroconductivity of PVP. Conversely, if ss-DNA probes bind to target miRNA, the AuNPs can’t interact with the DNA/miRNA hybrids formed because of its rigid structure. Hence, aggregated-AuNP are generated. These are concentrated in the sensing interface due to the strong interaction between AuNP and PVP assembled at the modified electrode surface via Au-N chemical bonds. The AuNP concentrated at the surface of the modified GCE electrocatalyze the oxidation of luminol which results in strong ECL. These findings were used to design a quantitative assay for trace levels of miRNA. To confirm the viability of the method, let-7a was used as a model analyte. Under the optimal conditions, the resulting calibration plot is linear in the 40 fM. to 1.0 pM concentration range with a detection limit as low as 20 fM. The method is even capable of discriminating most let-7 miRNA family members and was successfully applied to real sample assay. In our perception, the results demonstrate that this sensing interface represents a cost-effective and sensitive tool for determination of clinically relevant cancer biomarkers.

The electrochemiluminescent detection scheme for miRNAs is based on target-guided assembly of gold nanoparticles on a glassay carbon electrode modified with Nafion, carbon nanotubes and polyvinylpyrrolidone. The method is likely to have a wide scope in terms of gene expression profiling.





Similar content being viewed by others
References
MacRae I, Zhou K, Li F, Repic A, Brooks A, Cande W, Adams P, Doudna J (2006) Structural basis for double-stranded RNA processing by dicer. Science 311:195–198
Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X (2013) MicroRNA: function, detection, and Bioanalysis. Chem Rev 113:6207–6233
Cheng G (2015) Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev 81:75–93
Li Y, Liang L, Zhang C (2013) Isothermally sensitive detection of serum circulating miRNAs for lung cancer Diagosis. Anal Chem 85:11174–11179
Liu N, Olson E (2010) MicroRNA regulatory networks in cardiovascular development. Dev Cell 18:510–525
Cissell K, Deo S (2009) Trends in microRNA detection. Anal Bioanal Chem 394:1109–1116
Leshkowitz D, Horn-Saban S, Parmet Y, Feldmesser E (2013) Differences in microRNA detection levels are technology and sequence dependent. RNA 19:527–538
Va’lo’czi A, Hornyik C, Varga N, Burgya’n J, Kauppinenl S, Haveld Z (2004) Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res 32:e175
Pall G, Codony-Servat C, Byrne J, Ritchie L, Hamilton A (2007) Carbodiimide-mediated cross-linking of RNA to nylon membranes improves the detection of siRNA, miRNA and piRNA by northern blot. Nucleic Acids Res 35:e60
Chen C, Ridzon D, Broomer A, Zhou Z, Lee D, Nguyen J, Barbisin M, Xu N, Mahuvakar V, Andersen M, Lao K, Livak K, Karl G (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33:e179
Yu C, Yin B, Ye B (2013) A universal real-time PCR assay for rapid quantification of microRNAs via the enhancement of base-stacking hybridization. Chem Commun 49:8247–8249
Liu C, Calin G, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru C, Shimizu M, Zupo S, Dono M, Alder H, Bullrich F, Negrini M, and Croce C (2004) An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc. Natl. Acad. Sci. U.S.A 101: 9740–9744.
Lim L, Lau N, Garrett-Engele P, Grimson A, Schelter J, Castle J, Bartel D, Linsley P, Johnson J (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433:769–773
Teo A, Lim C, Gao Z (2014) The development of electrochemical assays for microRNAs. Electrochimi Acta 126:19–30
Jamali A, Pourhassan-Moghaddam M, Dolatabadi J, Omidi Y (2014) Nanomaterials on the road to microRNA detection with optical and electrochemical nanobiosensors. Trends Anal Chem 55:24–42
Miao W (2008) Electrogenerated Chemiluminescence and its Biorelated applications. Chem Rev 108:2506–2553
Hu L, Xu G (2010) Applications and trends in electrochemiluminescence. Chem Soc Rev 39:3275–3304
Zhang P, Wu X, Yuan R, Chai Y (2015) An “off-on” electrochemiluminescent biosensor based on DNAzyme-assisted target recycling and rolling circle amplifications for ultrasensitive detection of microRNA. Anal Chem 87:3202–3207
Zhang P, Zhuo Y, Chang Y, Yuan R, Chai Y (2015) Electrochemiluminescent Graphene quantum dots as a sensing platform: a dual amplification for MicroRNA assay. Anal Chem 87:10385–10391
Chen A, Gui G, Zhuo Y, Chai Y, Xiang Y, Yuan R (2015) Signal-off electrochemiluminescence biosensor based on Phi29 DNA polymerase mediated strand displacement amplification for MicroRNA detection. Anal Chem 87:6328–6334
Chen A, Ma S, Zhuo Y, Chai Y, Yuan R (2016) In situ electrochemical generation of electrochemiluminescent silver Naonoclusters on target-cycling synchronized rolling circle amplification platform for MicroRNA detection. Anal Chem 88:3203–3210
Cheng Y, Lei J, Chen Y, Ju H (2014) Highly selective detection of microRNA based on distance-dependent electrochemiluminescence resonance energy transfer between CdTe nanocrystals and Au nanoclusters. Biosens Bioelectron 51:431–436
Hao N, Li X, Zhang H, Xu J, Chen H (2014) A highly sensitive ratiometric electrochemiluminescent biosensor for microRNA detection based on cyclic enzyme amplification and resonance energy transfer. Chem Commun 50:14828–14830
Feng Q, Shen Y, Li M, Zhang Z, Zhao W, Xu J, Chen H (2016) Dual-wavelength electrochemiluminescence ratiometry based on resonance energy transfer between Au nanoparticles functionalized g-C3N4 Nanosheet and Ru(bpy)3 2+ for microRNA detection. Anal Chem 86:937–944
Zhang P, Wu X, Chai Y, Yuan R (2014) An electrochemiluminescent microRNA biosensor based on hybridization chain reaction coupled with hemin as the signal enhancer. Analyst 139(11):2748–2753
Liao Y, Huang R, Ma Z, Wu Y, Zhou X, Xing D (2014) Target-triggered enzyme-free amplification strategy for sensitive detection of MicroRNA in tumor cells and tissues. Anal Chem 86:4596–4604
Chili M, Pullabhotla V, Revaprasadu N (2011) Synthesis of PVP capped gold nanoparticles by the UV-irradiation technique. Mater Lett 65:2844–2847
Tang X, Jiang P, Ge G, Tsuji M, Xie S, Guo Y (2008) Poly(N-vinyl-2-pyrrolidone) (PVP)-capped dendritic gold nanoparticles by a one-step hydrothermal route and their high SERS effect. Langmuir 24:1763–1768
Zhang H, Okumura M, Toshima N (2011) Stable Disprsions of PVP-protected Au/Pt/Ag Trimetallic nanoparticles as highly active colloidal catalysts for aerobic glucose oxidation. J Phys Chem C 115:14883–14891
Song H, Rioux R, Hoefelmeyer J, Komor R, Niesz K, Grass M, Yang P, Somorjai G (2006) Hydrothermal growth of Mesoporous SBA-15 silica in the presence of PVP-stabilized Pt nanoparticles: synthesis, characterization, and catalytic properties. J Am Chem Soc 128:3027–3037
Liu X, Niu W, Li H, Han S, Hu L, Xu G (2008) Glucose biosensor based on gold nanoparticle-catalyzed luminol electrochemiluminescence on a three-dimensional sol-gel network. Electrochem Commun 10:1250–1253
Xu S, Liu Y, Wang T, Li J (2010) Highly sensitive Electrogenerated Chemiluminescence biosensor in profiling protein kinase activity and inhibition using gold nanoparticle as signal transduction probes. Anal Chem 82:9566–9572
Li Y, Tian R, Zheng X, Huang R (2016) Amplified electrochemical detection of nucleic acid hybridization via selective preconcentration of unmodified gold nanoparticles. Anal Chim Acta 934:59–65
Mucic R, Storhoff J, Mirkin C, Letsinger R (1998) DNA-directed synthesis of binary nanoparticle network materials. J Am Chem Soc 120:12674–12675
Li H and Rothberg L (2004) Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc. Natl. Acad. Sci. U.S.A 101: 14036–14039.
Li H, Rothberg L (2004) Label-free colorimetric detection of specific sequences in genomic DNA amplified by the polymerase chain reaction. J Am Chem Soc 126:10958–10961
Zhang Y, Yan Y, Chen W, Li S, Ding X, Li D, Wang H, Ju H, Ding S (2015) A simple electrochemical biosensor for highly sensitive and specific detection of microRNA based on mismatched catalytic hairpin assembly. Biosens Bioelectron 68:343–349
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21375085) and Foundation of Shaanxi Educational Department of China (16JK1131). The authors are grateful to Associate Professor Pan Wang from Shaanxi Normal University College of Life Sciences for providing breast cancer cell.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 4436 kb)
Rights and permissions
About this article
Cite this article
Xiong, H., Zheng, X. Electrochemiluminescence based determination of micro-RNA using target-guided assembly of gold nanoparticles on an electrode modified with Nafion, carbon nanotubes and polyvinylpyrrolidone. Microchim Acta 184, 1781–1789 (2017). https://doi.org/10.1007/s00604-017-2163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2163-x