Abstract
Aims
The addition of a single injection of insulin to the oral drugs (basal supported oral therapy; BOT) has been shown to greatly reduce blood glucose levels. The intermediate-acting NPH insulin (NPH) and the long-acting insulin glargine (Lantus®) have been compared for use in BOT in numerous clinical trials; however, their efficacy and safety in a real-life setting have not been described.
Methods
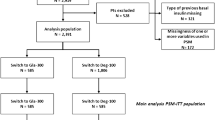
TIP (therapeutic benefits of patients on insulin glargine vs. NPH insulin being poorly controlled on prior short-time basal-insulin supported therapy with NPH insulin or insulin glargine) is a non-interventional, multicentre, observational study over 24 weeks. A total of 2629 patients were enrolled and 1931 were fully evaluable (1614 insulin glargine, 303 NPH insulin). Propensity scoring (PSM) was used to match 570 patients into 2 similar cohorts of 285 patients.
Results
In the PSM cohort, a slightly greater reduction in FBG and HbA1c levels was seen in the insulin glargine group compared to the NPH group. A weight loss, which was slightly more pronounced in insulin glargine patients despite receiving a lower insulin dose relative to the NPH group, was seen in both the groups. Additionally, hypoglycaemia, including nocturnal and severe events, was more prevalent in the patients receiving BOT with NPH. The occurrence of new micro- or macro-vascular complications and adverse events was low for both groups. A large proportion of patients changed from NPH therapy to insulin glargine therapy during the study, which was mainly attributable to insufficient glucose modulation. Improvements in quality of life and treatment satisfaction were found for both types of insulin.
Conclusions
This observational study provides evidence from a real-life setting that BOT with insulin glargine provides slightly greater reductions in weight, FBG and HbA1c levels, with a lower risk of hypoglycaemia than patients receiving NPH. This conclusion indicates that insulin glargine may be preferable to NPH insulin for BOT.


Similar content being viewed by others
References
Turner RC et al (1999) Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281(21):2005–2012
American Diabetes, A (2016) 7. Approaches to glycemic treatment. Diabetes Care 39(Suppl 1):S52–S59
Matthaei S et al (2009) Medical antihyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes 117(9):522–557
Home P et al (2014) Insulin therapy in people with type 2 diabetes: opportunities and challenges? Diabetes Care 37(6):1499–1508
Holman RR et al (2007) Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 357(17):1716–1730
Rys P et al (2015) Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol 52(4):649–662
Monami M et al (2016) Identification of predictors of response to basal insulin and DPP4 inhibitors in patients with type 2 diabetes failing to other therapies. Acta Diabetol 53(1):35–40
Baxter MA (2008) The role of new basal insulin analogues in the initiation and optimisation of insulin therapy in type 2 diabetes. Acta Diabetol 45(4):253–268
Yki-Jarvinen H et al (2000) Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 23(8):1130–1136
Yki-Jarvinen H (2002) Combination therapy with insulin and oral agents: optimizing glycemic control in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 18(Suppl 3):S77–S81
Mullins P et al (2007) Negative binomial meta-regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven Phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in type 1 and type 2 diabetes mellitus. Clin Ther 29(8):1607–1619
Rosenstock J et al (2005) Reduced hypoglycemia risk with insulin glargine: a meta-analysis comparing insulin glargine with human NPH insulin in type 2 diabetes. Diabetes Care 28(4):950–955
Moock J et al (2010) Development and Testing of the Insulin Treatment Experience Questionnaire (ITEQ). Patient 3(1):45–58
Massi Benedetti M et al (2003) A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with type 2 diabetes. Horm Metab Res 35(3):189–196
Fritsche A, Schweitzer MA, Haring HU (2003) Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 138(12):952–959
Lee P et al (2012) Comparison of safety and efficacy of insulin glargine and neutral protamine hagedorn insulin in older adults with type 2 diabetes mellitus: results from a pooled analysis. J Am Geriatr Soc 60(1):51–59
Riddle MC, Rosenstock J, Gerich J (2003) The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 26(11):3080–3086
Yki-Jarvinen H et al (2006) Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 49(3):442–451
Eliaschewitz FG et al (2006) Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch Med Res 37(4):495–501
Lepore M et al (2000) Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 49(12):2142–2148
Heinemann L et al (2000) Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 23(5):644–649
Linn T et al (2008) Nocturnal glucose metabolism after bedtime injection of insulin glargine or neutral protamine hagedorn insulin in patients with type 2 diabetes. J Clin Endocrinol Metab 93(10):3839–3846
Currie CJ, Johnson JA (2012) The safety profile of exogenous insulin in people with type 2 diabetes: justification for concern. Diabetes Obes Metab 14(1):1–4
Giugliano D et al (2011) Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7 % in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care 34(2):510–517
Hermansen K et al (2006) A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 29(6):1269–1274
Investigators OT et al (2012) Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 367(4):319–328
Pontiroli AE, Miele L, Morabito A (2011) Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 13(11):1008–1019
Igel LI et al (2016) Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep 18(4):16
Polonsky W et al (2014) More satisfied, but why? A pooled patient-level analysis of treatment satisfaction following the initiation of insulin glargine vs. comparators in insulin-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab 16(3):255–261
Petrovski G et al (2014) Successful desensitization in patient with type 2 diabetes with an insulin allergy using insulin pump and glargine. Acta Diabetol 51(6):1073–1075
Succurro E et al (2015) Bilateral lower limbs edema with “wooden” character induced by insulin glargine treatment. Acta Diabetol 52(4):809–811
Iafusco D et al (2015) Lower limbs edema by insulin glargine treatment: two other cases in pediatrics. Acta Diabetol. doi:10.1007/s00592-015-0797-x
Selam JL (2010) Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol 4(3):505–513
Asamoah E (2008) Insulin pen-the “iPod” for insulin delivery (why pen wins over syringe). J Diabetes Sci Technol 2(2):292–296
Korytkowski M et al (2003) A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther 25(11):2836–2848
Acknowledgments
The analysis was funded by Sanofi, Berlin, Germany.
Author contributions
HF designed the study. TW, AF, HF, and PB were involved in the analysis and interpretation of the data. PB drafted the first version of the manuscript, and all authors revised the article for important intellectual content. The final version was approved by all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TW and AF have attended advisory boards and received compensation from Sanofi Aventis Deutschland GmbH, Berlin, Germany. HF is an employee of Sanofi, Berlin, Germany. PB has received research funding and honoraria for consultancy from AstraZeneca, Bristol-Myers Squibb, Novartis, and Sanofi.
Ethical standard
The procedures in this study were in accordance with the ethical standards of the Declaration of Helsinki. The study protocol was approved by the ethics committee of the Berlin Chamber of Physicians. Furthermore the study was performed in accordance with the protocol, applicable local regulations and international guidelines.
Human and animal rights
All human rights were observed in keeping with the Declaration of Helsinki. There are no animal rights issues.
Informed consent
Written informed consent was obtained from all patients for being included in the study which has been done according to ethical standards and in keeping with the Declaration of Helsinki.
Additional information
Managed By Antonio Secchi.
Rights and permissions
About this article
Cite this article
Fiesselmann, A., Wiesner, T., Fleischmann, H. et al. Real-world therapeutic benefits of patients on insulin glargine versus NPH insulin. Acta Diabetol 53, 717–726 (2016). https://doi.org/10.1007/s00592-016-0862-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0862-0