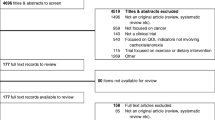
Abstract
Purpose
Previous studies reported promising efficacy for celecoxib in the treatment of cancer cachexia. We designed this study to test the hypothesis that combination therapy with megestrol acetate (MA) plus celecoxib is superior to MA alone.
Methods
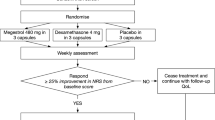
Ninety eligible gastrointestinal cancer patients randomly received either MA 320 mg/day plus placebo (arm1) or MA 320 mg/day plus celecoxib 200 mg/day (arm2). Patients were evaluated at baseline, then 1 and 2 months after starting interventions. The primary outcome was body weight. Secondary outcomes were quality of life, grip strength, appetite score, performance status, plasma albumin, CRP, IL-6, and Glasgow Prognostic Score.
Results
Patients were comparable at baseline. Sixty patients were assessable for the first month and 33 patients for the second month. After 2 months, patients in arm1 (MA + placebo) and arm2 (MA + celecoxib) experienced 4.0 ± 3.4 and 2.2 ± 3.6Kg of weight gain respectively (P = 0.163). Changes relative to baseline were statistically significant in both arms of the study (P = 0.001). Regarding secondary outcomes, comparisons between groups did not show any statistically significant difference, but within-group changes were significant in both arms of the study.
Conclusion
Since both MA alone and MA plus celecoxib are associated with improvement of cachexia in GI cancer patients, this study failed to show that adding celecoxib (200 mg/day) to megestrol (320 mg/day) could enhance anti-cachexic effects of megestrol.

Similar content being viewed by others
References
Hopkinson JB (2014) Psychosocial impact of cancer cachexia. J Cachexia Sarcopenia Muscle 5(2):89–94. https://doi.org/10.1007/s13539-014-0142-1
Fearon KC, Glass DJ, Guttridge DC (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16(2):153–166. https://doi.org/10.1016/j.cmet.2012.06.011
Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD (2008) Cachexia: a new definition. Clin Nutr (Edinburgh, Scotland) 27(6):793–799. https://doi.org/10.1016/j.clnu.2008.06.013
Ruiz Garcia V, Lopez-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S (2013) Megestrol acetate for treatment of anorexia-cachexia syndrome. The Cochrane database of systematic reviews (3):Cd004310.https://doi.org/10.1002/14651858.CD004310.pub3
Deans DA, Tan BH, Wigmore SJ, Ross JA, de Beaux AC, Paterson-Brown S, Fearon KC (2009) The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer 100(1):63–69. https://doi.org/10.1038/sj.bjc.6604828
McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12 (3):223–226. https://doi.org/10.1097/MCO.0b013e32832a7902
Rothwell NJ (1992) Eicosanoids, thermogenesis and thermoregulation. Prostaglandins Leukot Essent Fat Acids 46(1):1–7. https://doi.org/10.1016/0952-3278(92)90051-J
McCarthy DO, Whitney P, Hitt A, Al-Majid S (2004) Indomethacin and ibuprofen preserve gastrocnemius muscle mass in mice bearing the colon-26 adenocarcinoma. Res Nurs Health 27(3):174–184. https://doi.org/10.1002/nur.20019
Davis TW, Zweifel BS, O'Neal JM, Heuvelman DM, Abegg AL, Hendrich TO, Masferrer JL (2004) Inhibition of cyclooxygenase-2 by celecoxib reverses tumor-induced wasting. J Pharmacol Exp Ther 308(3):929–934. https://doi.org/10.1124/jpet.103.063099
Lai V, George J, Richey L, Kim HJ, Cannon T, Shores C, Couch M (2008) Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck 30(1):67–74. https://doi.org/10.1002/hed.20662
Mantovani G, Maccio A, Madeddu C, Serpe R, Antoni G, Massa E, Dessi M, Panzone F (2010) Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J Mol Med (Berlin, Germany) 88(1):85–92. https://doi.org/10.1007/s00109-009-0547-z
Madeddu C, Maccio A, Mantovani G (2012) Multitargeted treatment of cancer cachexia. Crit Rev Oncog 17(3):305–314. https://doi.org/10.1615/CritRevOncog.v17.i3.80
Madeddu C, Dessi M, Panzone F, Serpe R, Antoni G, Cau MC, Montaldo L, Mela Q, Mura M, Astara G, Tanca FM, Maccio A, Mantovani G (2012) Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib +/− megestrol acetate for patients with cancer-related anorexia/cachexia syndrome. Clin Nutr (Edinburgh, Scotland) 31(2):176–182. https://doi.org/10.1016/j.clnu.2011.10.005
Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, Cau MC, Panzone F, Mantovani G (2012) A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol 124(3):417–425. https://doi.org/10.1016/j.ygyno.2011.12.435
Mantovani G, Maccio A, Madeddu C, Serpe R, Massa E, Dessi M, Panzone F, Contu P (2010) Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 15(2):200–211. https://doi.org/10.1634/theoncologist.2009-0153
Mantovani G, Maccio A, Madeddu C, Gramignano G, Lusso MR, Serpe R, Massa E, Astara G, Deiana L (2006) A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiology, Biomarkers & Prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 15 (5):1030–1034.https://doi.org/10.1158/1055-9965.epi-05-0538
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495. https://doi.org/10.1016/s1470-2045(10)70218-7
Inui A (2005) Recent development in research and management of cancer anorexia-cachexia syndrome. Gan to Kagaku Ryoho Cancer Chemother 32(6):743–749
Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr (Edinburgh, Scotland) 29(2):154–159. https://doi.org/10.1016/j.clnu.2009.12.004
Argiles JM, Lopez-Soriano FJ, Toledo M, Betancourt A, Serpe R, Busquets S (2011) The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle 2(2):87–93. https://doi.org/10.1007/s13539-011-0027-5
Vigano AAL, Morais JA, Ciutto L, Rosenthall L, di Tomasso J, Khan S, Olders H, Borod M, Kilgour RD (2017) Use of routinely available clinical, nutritional, and functional criteria to classify cachexia in advanced cancer patients. Clin Nutr (Edinburgh, Scotland) 36(5):1378–1390. https://doi.org/10.1016/j.clnu.2016.09.008
LeBlanc TW, Nipp RD, Rushing CN, Samsa GP, Locke SC, Kamal AH, Cella DF, Abernethy AP (2015) Correlation between the international consensus definition of the cancer anorexia-cachexia syndrome (CACS) and patient-centered outcomes in advanced non-small cell lung cancer. J Pain Symptom Manag 49(4):680–689. https://doi.org/10.1016/j.jpainsymman.2014.09.008
Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, Fearon K, Strasser F, Kaasa S (2014) Validation of the consensus-definition for cancer cachexia and evaluation of a classification model—a study based on data from an international multicentre project (EPCRC-CSA). Ann Oncol 25(8):1635–1642. https://doi.org/10.1093/annonc/mdu086
Wallengren O, Lundholm K, Bosaeus I (2013) Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer : Off J Multinatl Assoc Support Care Cancer 21(6):1569–1577. https://doi.org/10.1007/s00520-012-1697-z
Gebbia V, Testa A, Gebbia N (1996) Prospective randomised trial of two dose levels of megestrol acetate in the management of anorexia-cachexia syndrome in patients with metastatic cancer. Br J Cancer 73(12):1576–1580. https://doi.org/10.1038/bjc.1996.297
De Conno F, Martini C, Zecca E, Balzarini A, Venturino P, Groff L, Caraceni A (1998) Megestrol acetate for anorexia in patients with far-advanced cancer: a double-blind controlled clinical trial. Eur J Cancer 34(11):1705–1709. https://doi.org/10.1016/S0959-8049(98)00219-6
Loprinzi CL, Bernath AM, Schaid DJ, Malliard JA, Athmann LM, Michalak JC, Tschetter LK, Hatfield AK, Morton RF (1994) Phase III evaluation of 4 doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. Oncology 51(Suppl 1):2–7. https://doi.org/10.1159/000227407
Vadell C, Segui MA, Gimenez-Arnau JM, Morales S, Cirera L, Bestit I, Batiste E, Blanco R, Jolis L, Boleda M, Anton I (1998) Anticachectic efficacy of megestrol acetate at different doses and versus placebo in patients with neoplastic cachexia. Am J Clin Oncol 21(4):347–351. https://doi.org/10.1097/00000421-199808000-00006
Ulutin HC, Arpaci F, Pak Y (2002) Megestrol acetate for cachexia and anorexia in advanced non-small cell lung cancer: a randomized study comparing two different doses. Tumori 88(4):277–280
Savides TJ, Jensen DM, Cohen J, Randall GM, Kovacs TO, Pelayo E, Cheng S, Jensen ME, Hsieh HY (1996) Severe upper gastrointestinal tumor bleeding: endoscopic findings, treatment, and outcome. Endoscopy 28(2):244–248. https://doi.org/10.1055/s-2007-1005436
Moreno-Otero R, Rodriguez S, Carbo J, Mearin F, Pajares JM (1987) Acute upper gastrointestinal bleeding as primary symptom of gastric carcinoma. J Surg Oncol 36(2):130–133. https://doi.org/10.1002/jso.2930360212
Mantovani G, Madeddu C, Maccio A, Gramignano G, Lusso MR, Massa E, Astara G, Serpe R (2004) Cancer-related anorexia/cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Cancer Epidemiol, Biomark Prev : Publ Am Assoc Cancer Res, cosponsored by the American Society of Preventive Oncology 13(10):1651–1659
Hefler-Frischmuth K, Seebacher V, Polterauer S, Tempfer C, Reinthaller A, Hefler L (2010) The inflammation-based modified Glasgow Prognostic Score in patients with vulvar cancer. Eur J Obstet Gynecol Reprod Biol 149(1):102–105. https://doi.org/10.1016/j.ejogrb.2009.12.027
NCI, NIH, DHHS (May 29, 2009) common terminology criteria for adverse events v4.0 vol 09-5410. NIH publication United States
McMillan DC, Wigmore SJ, Fearon KC, O'Gorman P, Wright CE, McArdle CS (1999) A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer 79(3–4):495–500. https://doi.org/10.1038/sj.bjc.6690077
Cerchietti LC, Navigante AH, Peluffo GD, Diament MJ, Stillitani I, Klein SA, Cabalar ME (2004) Effects of celecoxib, medroxyprogesterone, and dietary intervention on systemic syndromes in patients with advanced lung adenocarcinoma: a pilot study. J Pain Symptom Manag 27(1):85–95. https://doi.org/10.1016/j.jpainsymman.2003.05.010
von Haehling S, Anker SD (2010) Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 1(1):1–5. https://doi.org/10.1007/s13539-010-0002-6
Tuca A, Jimenez-Fonseca P, Gascon P (2013) Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol 88(3):625–636. https://doi.org/10.1016/j.critrevonc.2013.07.015
Rogers ES, MacLeod RD, Stewart J, Bird SP, Keogh JW (2011) A randomised feasibility study of EPA and Cox-2 inhibitor (Celebrex) versus EPA, Cox-2 inhibitor (Celebrex), resistance training followed by ingestion of essential amino acids high in leucine in NSCLC cachectic patients—ACCeRT study. BMC Cancer 11(1):493. https://doi.org/10.1186/1471-2407-11-493
Cohen JS (2005) How celecoxib could be safer, how valdecoxib might have been. Ann Pharmacother 39(9):1542–1545. https://doi.org/10.1345/aph.1G175
Singh G, Fort JG, Goldstein JL, Levy RA, Hanrahan PS, Bello AE, Andrade-Ortega L, Wallemark C, Agrawal NM, Eisen GM, Stenson WF, Triadafilopoulos G (2006) Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am J Med 119(3):255–266. https://doi.org/10.1016/j.amjmed.2005.09.054
de Souza CO, Kurauti MA, de Fatima Silva F, de Morais H, Borba-Murad GR, de Andrade FG, de Souza HM (2015) Effects of celecoxib and ibuprofen on metabolic disorders induced by Walker-256 tumor in rats. Mol Cell Biochem 399(1–2):237–246. https://doi.org/10.1007/s11010-014-2250-9
Buskermolen S, Langius JA, Kruizenga HM, Ligthart-Melis GC, Heymans MW, Verheul HM (2012) Weight loss of 5% or more predicts loss of fat-free mass during palliative chemotherapy in patients with advanced cancer: a pilot study. Nutr Cancer 64(6):826–832. https://doi.org/10.1080/01635581.2012.690062
Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM (2005) Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut 54(4):540–545. https://doi.org/10.1136/gut.2004.047563
Ordu C, Pilanci KN, Koksal UI, Okutur K, Saglam S, Tecimer C, Demir G (2014) Can megestrol acetate induce thrombosis in advanced oncology patients receiving chemotherapy? Asian Pac J Cancer Prev : APJCP 15(23):10165–10169
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol was approved by Mazandaran University of Medical Sciences Ethical Committee (IR.MAZUMS.REC.94-1087) and registered in Iranian Registry of Clinical Trials (IRCT201407222027N4). All patients gave written informed consent before inclusion into the study.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(SAV 67 kb)
Rights and permissions
About this article
Cite this article
Kouchaki, B., Janbabai, G., Alipour, A. et al. Randomized double-blind clinical trial of combined treatment with megestrol acetate plus celecoxib versus megestrol acetate alone in cachexia-anorexia syndrome induced by GI cancers. Support Care Cancer 26, 2479–2489 (2018). https://doi.org/10.1007/s00520-018-4047-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4047-y