Abstract
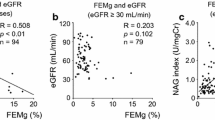
Urinary microalbumin excretion was assessed in 76 children with asymptomatic microscopic hematuria in whom the presence of proteinuria, hypertension, reduced renal function, hypercalciuria, urinary tract infection or structural abnormality of the urinary tract had been excluded. All children underwent a percutaneous kidney biopsy to determine whether microalbumin excretion can be used as a marker to predict the source of hematuria. Microalbumin excretion was considered normal if the urinary ratio of microalbumin to creatinine (MA/Cr ug/mg) was ≤30. Twenty-two (29%) had microalbuminuria (MA/Cr 96±30 ug/mg) and 54 (71%) had normal albumin excretion (MA/Cr 13±2 ug/mg). Of those with normoalbuminuria, 38 (70%) had normal renal tissue, 15 (28%) thin glomerular basement membrane (TGBM) disease and 1 (2%) IgA nephropathy. In contrast, 20 (91%) of those with microalbuminuria had IgA nephropathy and 2 (9%) had TGBM disease. The mean urinary MA/Cr ratio for all IgA children was 89±32 ug/mg higher compared with a value for the children with TGBM disease (14 ±3 ug/mg, P <0.001) or children whose renal biopsy appeared normal (11±2 ug/mg, P <0.001). Statistical analysis revealed no significant differences between the mean MA/Cr ug/mg ratio for children with TGBM disease and those with normal glomerular findings. Fourteen of the 20 children with IgA nephropathy who also had microalbuminuria were treated with an angiotensin-converting enzyme (ACE) inhibitor. Over a mean follow-up of 51 months, none developed overt proteinuia; hematuria resolved and microalbuminuria returned to normal in eight (57%) during therapy with the ACE-inhibitor. In contrast, hematuria persisted and prtoteinuria developed in the other untreated children. None of the children with TGBM disease developed overt proteinuria after a mean of 51 months. Hematuria was persistent in children with TGBM disease, but often resolved in those whose biopsies were completely normal. These data suggest that determination of urinary microalbumin excretion is warranted in the routine examination of children with isolated microscopic hematuria. Routine screening for microalbuminuria may help to identify a subgroup of patients with IgA nephropathy who are at high risk for progressive kidney disease and need more intensive therapy and closer follow-up.
Similar content being viewed by others
References
Vehaskari VM, Rapola J, Koskimies O, Savilahti E, Vilska J, Hallman N (1979) Micrsocopic hematuria in school children: epidemiology and clinicopathologic evaluation. J Pediatr 95:676–684
Feld LG, Waz Wr, Perez LM, Joseph DB (1997) Hematuria. An Integrated medical and surgical approach. Pediatr Clin North Am 44:1191-1210
D’Amico G (1987) The commonest glomerulonephritis in the world: IgA nephropathy. Quart J Med 64:709–727
Yoshikawa N, Iijima K, Ito H (1999) IgA nephropathy in children. Nephron 83:1–12
Vehaskari VM (1982) Asymptomatic hematuria-A cause for concern? Pediatr Nephrol 3:240–241
Blumenthal SS, Fritsche C, Lemann J Jr (1988) Establishing the diagnosis of benign familial hematuria. The importance of examining the urine sediment of family members. JAMA 15:2263–2266
Kashton CE (1998) Alport syndrome and thin glomerular basement membrane disease. J Am Soc Nephrol 9:1736-1750
Trachtman H, Weiss RA, Bennett B, Greiffer I (1984) Isolated hematuria in children. Indication for a renal biopsy. Kidney Int 25:94–99
Sinniah R, Pwee Hs, LIM CM (1976) Glomerular lesions in asymptomatic microscopic hematuria discovered on routine medical examination. Clin Nephrol 5:216–228
Cohen AH, Nast CC, Adler SG, Kopple JD (1989) Clinical utility of kidney biopsies in the diagnosis and management of renal disease. Am J Nephrol 9:309–315
Madaio MP (1990) Renal biopsy. Kidney Int 38:529–543
Chiarelli F, Verrotti A, Morgese G (1995) Glomerular hyperfiltration increases the risk of developing microalbuminuria in diabetic children. Pediatr Nephrol 9:154–158
Assadi FK (2002) Quantitation of microalbuminuria using random urine samples. Pediat Nephrol 17:107–110
Shihabi ZK, Konen JC, O’Conner ML (1991) Albuminuria vs. urinary total protein for detecting chronic renal disorders. Clin Chem 37:621–624
Diven SC, Travis LB (2000) A practical primary care approach to hematuria in children. Pediatr Nephrol 14:65-72
Stapleton FB, Roy S III, Noe HN, Jerkins G (1984) Hypercalciuria in children with hematuria. N Engl J Med 310:1345–1348
Nathan DM, Rosenbaum C, Protasowicki VD (1987) Single-voided samples can be used to estimate quantitative proteinuria. Diabetes Care 10:414–418
Heinegard D, Tiderstrom G (1973) Determination of serum creatinine by a direct colorimetric method. Clin Chem Acta 43:305–310
Keane WF, Eknoyan G (1999) Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 33:1004–1010
McLay ALC, Jackson R, Meyboom F, Bouton Jones JM (1992) Glomerular basement membrane thinning in adults.Clinicopathological correlations of a new diagnostic approach. Nephrol Dial Transplant 7:191-199
Dische FE (1992) Measurement of glomerular basement membrane thickness and its diagnosis of thin-basement membrane nephropathy. Arch Pathol Laboratory Med 116:43–49
Messent JW, Elliot TG, Hill RD, Jarret RJ, Keen H, Viberti GC (1992) Prognostic significance of microalbuminuria in insulin-dependent diabetes mellitus: a twenty-three year follow-up study. Kidney Int 41:836–839
Hong KS, Kim SY, Koo WS, Chai EJ, Cha BY, Chang YS, Yoon YS, Son HY, Bang BK (1988) The clinical utility of microalbuminuria in nephrotic syndrome with complete remission, isolated microscopic hematuria and renal transplantation donors. Korean J Intern Med 3:117–121
Eardley KS, Ferreira MAS, Howie AJ, Gosling P, Lipkin GW (2004) Urinary albumin excretion: a predictor of glomerular findings in adults with microscopic hematuria. QJ Med 97:297–301
Galla J (2001) Molecular genetics in IgA nephropathy. Nephron 88:107–112
Lemmink HH, Nilesen WN, Mochizuki T, Schroder CH, Brunner HG, vanOost BA, Monnens LA, Smeets HJ (1996) Benign familial hematuria due to mutation of the type IV collagen alpha 4 gene. J Clin Invst 98:1114–1118
Badenas C, Praga M, Tazon B Heidet L, Arrondel C, Armengol A, Andress A, Morales A, Camachio JA, Lens X, Davila S, Mila M, Antignac C, Darnell A, Torra (2002) Mutations in the COL4A4 and COL4A3 genes causes familial benign hematuria. J Am Soc Nephrol 13:1248–1254
Buzza M, Wilson D, Savige J (2001) Segregation of hematuria in thin basement membrane disease with haplotypes at the loci for Alport syndrome. Kidney Int 59:1670–1676
Tiebosch AT, Feredeik PM, van Breda Vriesman PJ (1989) Thin-basement- membrane nephropathy in adults with persistent hematuria. N Engl J Med 320:14–18
Perry GJ, Georgr CR, Field MJ Collet PV, Kalowski S, Wyndham RN, Newland RC, Lin BP, Kneale KL, Lawrence JR (1989) Thin-membrane nephropathy-a common cause of glomerular hematuria Med J Aus 151:638–642
Cosio FG, Falkenhain ME, Sedmak DD (1994) Association of thin glomerular basement membrane with other glomerulopathies. Kidney Int 46:471–474
Endo M, Ohi H, Satomura A (2001) Regulation of in situ complement activation via the lectin pathway in patients with IgA nephropathy. Clin Nephrol 55:185–191
Janssen U, Bahlmann F Kohl J, Zwirner J, Haubitz M, Floege J (2000) Activation of acute phase response and C3 in patients with IgA nephropathy. Am J Kidney Dis 35:21–28
Reddan D, Owen W (2001) IgA nephropathy and inhibition of the renin-angiotensin system: Is reduction of proteiuria adequate proof of efficiency? Am J Kidney Dis 38:182–185
Feehally J, O’Donoghue DJ, Ballardie FW (1989) Current nephrological practice in the investigation of hematuria: relationship to incidence of IgA nephropathy. Kidney Int 49:222–225
Nolin L, Courteau M (1999) Management of IgA nephropathy: Evidence-based recommendations. Kidney Int 55:S56–S62
Szeto CC, Lai FM, To KF, Wong TY, Chew KM, Choi PC, Lui SF, Li PK (2001) The natural history of immunoglobulin A nephropathy among patients with hematuria and minimal proteinuria. Am J Med 11:434–437
Hunsicker LG, Atkins RC, Lewis JB, Braden G, de Cresoigny PJ, Defer G, Drury P, Locatelli F, Wiegmann TB, Lewis EJ, for the Collaborative Study Group (2004) Impact of irbesartan, blood pressure control, and proteinuria on renal outcomes in the irbesartan diabetic nephropathy trial. Kidney Int [Suppl] 2:S99–S101
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz J, Atkins RC, Rohde R, Raz I; for Collaborative Study Group (2001) Renoprotectve effect of the angiotensin-receptor-antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 20:851–860
Wyatt RJ, Hogg RJ (2001) Evidence-based assessment of treatment options for children with IgA nephropathy. Pediatr Nephrol 16:156–167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assadi, F.K. Value of urinary excretion of microalbumin in predicting glomerular lesions in children with isolated microscopic hematuria. Pediatr Nephrol 20, 1131–1135 (2005). https://doi.org/10.1007/s00467-005-1928-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-1928-3