Abstract
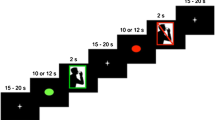
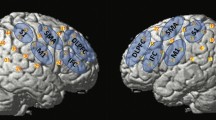
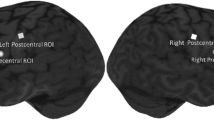
Esophageal acid exposure induces sensory and motility changes in the upper gastrointestinal tract; however, the mechanisms involved and the effects on activity in the brain regions that control swallowing are unknown. The aim of this study was to examine functional changes in the cortical swallowing network as a result of esophageal acidification using functional magnetic resonance imaging (fMRI). Seven healthy volunteers (3 female, age range = 20–30 years) were randomized to receive either a 0.1 M hydrochloric acid or (control) saline infusion for 30 min into the distal esophagus. Postinfusion, subjects underwent four 8 min blocks of fMRI over 1 h. These alternated between 1 min swallowing water boluses and 1 min rest. Three-dimensional cluster analysis for group brain activation during swallowing was performed together with repeated-measures ANOVA for differences between acid and saline. After acid infusion, swallowing-induced activation was seen predominantly in postcentral gyrus (p < 0.004). ANOVA comparison of acid with saline showed a significant relative reduction in activation during swallowing of the precentral gyrus (M1) BA 4 (p < 0.008) in response to acid infusion. No areas of increased cortical activation were identified with acid vs. saline during swallowing. Esophageal acidification inhibits motor and association cortical areas during a swallowing task, probably via changes in vagal afferent or nociceptive input from the esophagus. This mechanism may play a protective role, facilitating acid clearance by reduced descending central motor inhibition of enteric/spinal reflexes, or by preventing further ingestion of injurious agents.

Similar content being viewed by others
References
Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res 2001;140(3):280–289.
Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci 1998;1(1):64–68.
Fraser C, Rower M, Hamdy S, Rothwell J, Hobday D, Hollander I, Tyrell P, Hobson A, Williams S. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 2002;34(5):831–840.
Gow D, Hobson AR, Furlong P, Hamdy S. Characterising the central mechanisms of sensory modulation in human swallowing motor cortex. Clin Neurophysiol 2004;115(10):2382–2390.
Holloway RH. Esophageal body motor response to reflux events: secondary peristalsis. Am J Med 2000;108(Suppl 4a):20S–26S.
Helm JF, Dodds WJ, Pelc LR, Palmer DW, Hogan WJ, Teeter BC. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. N Engl J Med 1984;310(5):284–288.
Brown CM, Snowdon CF, Slee B, Sandle LN, Rees WD. Effect of topical oesophageal acidification on human salivary and oesophageal alkali secretion. Gut 1995;36(5):649–653.
Smit CF, van Leeuwen JA, Mathus-Vliegen LM, Devriese PP, Semin A, Tan J, Schouwenburg PF. Gastropharyngeal and gastroesophageal reflux in globus and hoarseness. Arch Otolaryngol Head Neck Surg 2000;126(7):827–830.
Knight RE, Wells JR, Parrish RS. Esophageal dysmotility as an important co-factor in extraesophageal manifestations of gastroesophageal reflux. Laryngoscope 2000;110(9):1462–1466.
Grossi L, Ciccaglione AF, Travaglini N, Marzio L, Swallows, oesophageal and gastric motility in normal subjects and in patients with gastro-oesophageal reflux disease: a 24-h pH-manometric study. Neurogastroenterol Motil 1998;10(2):115–121.
Castell DO, Murray JA, Tutuian R, Orlando RC, Arnold R. Review article: the pathophysiology of gastro-oesophageal reflux disease-oesophageal manifestations. Aliment Pharmacol Ther 2004;20(Suppl 9):14–25.
Lundell L, Myers JC, Jamieson GG. Is motility impaired in the entire upper gastrointestinal tract in patients with gastro-oesophageal reflux disease? Scand J Gastroenterol 1996;31(2):131–135.
Crozier RE, Glick ME, Gibb ME, Gibb SP, Ellis FH Jr, Veerman JM. Acid-provoked esophageal spasm as a cause of noncardiac chest pain. Am J Gastroenterol 1991;86(11):1576–1580.
Sarkar S, Aziz Q, Woolf CJ, Hobson AR, Thompson DG. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet 2000;356(9236):1154–1159.
Atkinson M, Bennett JR. Relationship between motor changes and pain during esophageal acid perfusion. Am J Dig Dis 1968;13(4):346–350.
Garabedian M. Uses of esophageal manometry and acid perfusion in the study of gastroesophageal reflux and hiatal hernia. Surg Clin North Am 1971;51(3):589–596.
Wallin L, Boesby S, Madsen T. The effect of HCl infusion in the lower part of the oesophagus on the pharyngo-oesophageal sphincter pressure in normal subjects. Scand J Gastroenterol 1978;13(7):821–826.
Madsen T, Wallin L, Boesby S, Larsen VH. Spontaneous peristaltic activity in the oesophagus after imitated acid gastro-oesophageal reflux. A study in normal subjects. Scand J Gastroenterol 1982;17(6):811–815.
Malmud LS, Fisher RS. Radionuclide studies of esophageal transit and gastroesophageal reflux. Semin Nucl Med 1982;12(2):104–115.
Madsen T, Wallin L, Boesby S, Larsen VH. Oesophageal peristalsis in normal subjects. Influence of pH and volume during imitated gastro-oesophageal reflux. Scand J Gastroenterol 1983;18(4):513–518.
Burns TW, Venturatos SG. Esophageal motor function and response to acid perfusion in patients with symptomatic reflux esophagitis. Dig Dis Sci 1985;30(6):529–535.
Kjellen G, Tibbling L. Oesophageal motility during acid-provoked heartburn and chest pain. Scand J Gastroenterol 1985;20(8):937–940.
Katz PO, Dalton CB, Richter JE, Wu WC, Castell DO. Esophageal testing of patients with noncardiac chest pain or dysphagia. Results of three years’ experience with 1161 patients. Ann Intern Med 1987;106(4):593–597.
Thompson DG, Andreollo NA, McIntyre AS, Earlan RJ. Studies of the oesophageal clearance responses to intraluminal acid. Gut 1988;29(7):881–885.
Andreollo NA, Thompson DG, Kendall GP, McIntyre AS, Earlam RJ. Motor responses of the upper esophageal sphincter and body to intraluminal acid. Braz J Med Biol Res 1989;22(1):51–60.
Helm JF, Massey BT, Martin CJ, Dodds WJ, Hogan WJ, Arndorfer RC. Oesophageal acidification does not increase lower oesophageal sphincter pressure. Gut 1990;31(3):266–269.
Bontempo I, Piretta L, Corazziari E, Michetti F, Anzini F, Torsoli A. Effects of intraluminal acidification on oesophageal motor activity. Gut 1994;35(7):884–890.
Anggiansah A, Taylor G, Marshall RE, Bright NF, Owen WA, Owen WJ. Oesophageal motor responses to gastro-oesophageal reflux in healthy controls and reflux patients. Gut 1997;41(5):600–605.
McDougall NI, Mooney RB, Ferguson WR, Collins JS, McFarland RJ, Love AH. The effect of healing oesophagitis on oesophageal motor function as determined by oesophageal scintigraphy and ambulatory oesophageal motility/pH monitoring. Aliment Pharmacol Ther 1998;12(9):899–907.
Simren M, Silny J, Holloway R, Tack J, Janssens J, Sifrim D. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut 2003;52(6):784–790.
Bhalla V, Liu J, Puckett JL, Mittal RK. Symptom hypersensitivity to acid infusion is associated with hypersensitivity of esophageal contractility. Am J Physiol Gastrointest Liver Physiol 2004;287(1):G65–G71.
Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology 1998;114(3):559–578.
Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson dG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology 2004;126(3):683–692.
Sarkar S, Hobson AR, Hughes A, Growcott J, Woolf CJ, Thompson DG, Aziz Q. The prostaglandin E2 receptor-1 (EP-1) mediates acid-induced visceral pain hypersensitivity in humans. Gastroenterology 2003;124(1):18–25.
Sarkar S, Hobson AR, Furlong PL, Woolf cJ, Thompson DG, Aziz Q. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2001;281(5):G1196–G202.
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med 1996;35(3):346–355.
Bullmore ET, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SC, Sharma T, McGuire PK. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 1999;7(1):38–48.
Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage 1998;7(1):30–40.
Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp 2001;12(2):61–78.
Talairach J, Tournoux P, Musolino A, Missir O. Stereotaxic exploration in frontal epilepsy. Adv Neurol 1992;57:651–688.
Kern M, Hoffman C, Hyde J, Shaker R. Characterization of the cerebral cortical representation of heartburn in GERD patients. Am J Physiol Gastrointest Liver Physiol 2004;286(1):G174–G181.
Lang IM, Dean C, Medda BK, Aslam M, Shaker R. Differential activation of medullary vagal nuclei during different phases of swallowing in the cat. Brain Res 2004;1014(1–2):145–163.
Kern M, Lawal A, Sanjeevi A, Hyde J, Shaker R. Confirmation of neural hypersensitivity following central sensitisation by fMRI recording of subliminal esophageal distension. Gastroenterology 2005; 128(4 Suppl 2): abstract M1591.
Kern MK, Birn RM, Jaradeh S, Jesmanowicz A, Cox RW, Hyde JS, Shaker R. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology 1998;115(6):1353–1362.
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol 1999;277(1 Pt 1):G219–G225.
Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 1999;109(9):1417–1423.
Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 2001;85(2):938–950.
Hobson AR, Khan RW, Sarkar S, Rurlong PL, Aziz Q. Development of esophageal hypersensitivity following experimental duodenal acidification. Am J Gastroenterol 2004;99(5):813–820.
Broussard DL, Lynn RB, Wiedner EB, Altschuler SM. Solitarial premotor neuron projections to the rat esophagus and pharynx: implications for control of swallowing. Gastroenterology 1998;114(6):1268–1275.
Farina S, Tinazzi M, LePera D, Valeriani M. Pain-related modulation of the human motor cortex. Neurol Res 2003;25(2):130–142.
Drewes AM, Reddy H, Staahl C, Pedersen J, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Sensory-motor responses to mechanical stimulation of the esophagus after sensitization with acid. World J Gastroenterol 2005;11(28):4367–4374.
Lang IM, Medda BK, Gray M, Shaker R. Esophageal acidification and distension activate different sets of medullary vagal nuclei. Gastroenterology 2005;128(4 Suppl 2): abstract M1595.
Shuai XW, Xie PY. Expression and localization of c-Fos and NOS in the central nerve system following esophageal acid stimulation in rats. World J Gastroenterol 2004;10(15):2287–2291.
Suwanprathes P, Ngu M, Ing A, Hunt G, Seow F. c-Fos immunoreactivity in the brain after esophageal acid stimulation. Am J Med 2003;115(Suppl 3A):31S–38S.
Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, George MS. A review of functional neuroimaging studies of vagus nerve stimulation (VNS). J Psychiatr Res 2003;37(6):443–455.
Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J Neurophysiol 1999;81(4):1917–1926.
Acknowledgments
P. Paine was funded by the Wellcome Trust (UK). The authors thank the MR radiography staff for their invaluable assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Studies were performed in the Department of GI Sciences and Translational Imaging Unit, University of Manchester, UK
Rights and permissions
About this article
Cite this article
Paine, P.A., Hamdy, S., Chitnis, X. et al. Modulation of Activity in Swallowing Motor Cortex Following Esophageal Acidification: A Functional Magnetic Resonance Imaging Study. Dysphagia 23, 146–154 (2008). https://doi.org/10.1007/s00455-007-9114-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-007-9114-3