Abstract
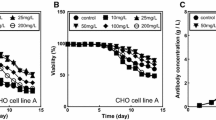
The effects of all-trans retinoic acid (RA) and sodium butyrate (NaBu) on growth, viability and antibody production of two types of transfected Chinese hamster ovary cell lines (CHO-K1 and CHO-S) were investigated using a batch mode cell culture. By adding 0.5 mM NaBu in the CHO-K1 cell culture, the cell specific productivity (Qp) and antibody concentration increased by five- and threefold, respectively. The optimal concentration of RA was 100 nM which resulted in twofold increase in antibody production. In a combination model, RA applied at early growth phase of CHO-K1 cells followed by addition of NaBu with lowering culture temperature at the end of stationary phase resulted in two- and threefold increase in Qp and final antibody concentration, respectively. The latter strategy was also applied on suspended CHO-S cells with enhanced Qp and antibody concentration, but to a lesser extent than the CHO-K1 cells. In conclusion, our results demonstrate that the addition of RA and NaBu along with lowering the culture temperature can increase cell culture period as well as Qp and the final concentration of recombinant monoclonal antibody in both CHO-K1 and CHO-S cells without any significant change in binding affinity of the mAb.






Similar content being viewed by others
References
Walsh G (2014) Biopharmaceutical benchmarks 2014. Nat Biotechnol 32(10):992–1000. https://doi.org/10.1038/nbt.3040
Konno Y, Aoki M, Takagishi M, Sakai N, Koike M, Wakamatsu K, Hosoi S (2011) Enhancement of antibody production by the addition of Coenzyme-Q(10). Cytotechnology 63(2):163–170. https://doi.org/10.1007/s10616-010-9330-9
Choi SS, Rhee Wj Fau - Kim EJ, Kim Ej Fau -. Park TH, Park TH (2006) Enhancement of recombinant protein production in Chinese hamster ovary cells through anti-apoptosis engineering using 30Kc6 gene. Biotechnol Bioeng 95(3):459–467. https://doi.org/10.1002/bit.21023
Chen K, Liu Q, Xie L, Sharp PA, Wang DI (2001) Engineering of a mammalian cell line for reduction of lactate formation and high monoclonal antibody production. Biotechnol Bioeng 72 (1):55–61. https://doi.org/10.1002/1097-0290(20010105)72:1<55::AID-BIT8>3.0.CO;2-4
Kim DY, Lee JF, Chang HN, Chang Hn Fau - Oh DJ, Oh DJ (2005) Effects of supplementation of various medium components on chinese hamster ovary cell cultures producing recombinant antibody. In: Cytotechnology (0920–9069 (Print)):37–49. doi:D-NLM: PMC3449820 EDAT-2008/11/13 09:00 MHDA-2008/11/13 09:01 CRDT-2008/11/13 09:00 PHST-2005/03/31 [received] PHST-2005/07/29 [accepted] AID—https://doi.org/10.1007/s10616-005-3775-2. PST-publish
Sun Y-t, Zhao L, Ye Z, Fan L, Liu X-p, Tan W-S (2013) Development of a fed-batch cultivation for antibody-producing cells based on combined feeding strategy of glucose and galactose. Biochem Eng J 81:126–135. https://doi.org/10.1016/j.bej.2013.10.012
Kumar N, Gammell P, Clynes M (2007) Proliferation control strategies to improve productivity and survival during CHO based production culture: a summary of recent methods employed and the effects of proliferation control in product secreting CHO cell lines. Cytotechnology 53(1–3):33–46. https://doi.org/10.1007/s10616-007-9047-6
Sunley K, Butler M (2010) Strategies for the enhancement of recombinant protein production from mammalian cells by growth arrest. Biotechnol Adv 28(3):385–394. https://doi.org/10.1016/j.biotechadv.2010.02.003
Park JH, Noh SM, Woo JR, Kim JW, Lee GM (2016) Valeric acid induces cell cycle arrest at G1 phase in CHO cell cultures and improves recombinant antibody productivity. Biotechnol J 11(4):487–496. https://doi.org/10.1002/biot.201500327
Ahn WS, Jeon J-J, Jeong Y-R, Lee SJ, Yoon SK (2008) Effect of culture temperature on erythropoietin production and glycosylation in a perfusion culture of recombinant CHO cells. Biotechnol Bioeng 101(6):1234–1244. https://doi.org/10.1002/bit.22006
Chen Z-L, Wu B-C, Liu H, Liu X-M, Huang P-T (2004) Temperature shift as a process optimization step for the production of pro-urokinase by a recombinant Chinese hamster ovary cell line in high-density perfusion culture. J Biosci Bioeng 97(4):239–243. https://doi.org/10.1016/S1389-1723(04)70198-X
Allen MJ, Boyce JP, Trentalange MT, Treiber DL, Rasmussen B, Tillotson B, Davis R, Reddy P (2008) Identification of novel small molecule enhancers of protein production by cultured mammalian cells. Biotechnol Bioeng 100(6):1193–1204. https://doi.org/10.1002/bit.21839
Bi J-X, Shuttleworth J, Al-Rubeai M (2004) Uncoupling of cell growth and proliferation results in enhancement of productivity in p21CIP1-arrested CHO cells. Biotechnol Bioeng 85(7):741–749. https://doi.org/10.1002/bit.20025
Wurm MF (2013) CHO quasispecies—implications for manufacturing processes. Processes. https://doi.org/10.3390/pr1030296
Yoon SK, Hong JK, Lee GM (2004) Effect of simultaneous application of stressful culture conditions on specific productivity and heterogeneity of erythropoietin in chinese hamster ovary cells. Biotechnol Prog 20(4):1293–1296. https://doi.org/10.1021/bp034382z
Hendrick V, Winnepenninckx P, Abdelkafi C, Vandeputte O, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G, Werenne J (2001) Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology 36(1–3):71–83. https://doi.org/10.1023/A:1014088919546
Hong JK, Lee SM, Kim K-Y, Lee GM (2014) Effect of sodium butyrate on the assembly, charge variants, and galactosylation of antibody produced in recombinant Chinese hamster ovary cells. Appl Microbiol Biotechnol 98(12):5417–5425. https://doi.org/10.1007/s00253-014-5596-8
Hong JK, Lee GM, Yoon SK (2011) Growth factor withdrawal in combination with sodium butyrate addition extends culture longevity and enhances antibody production in CHO cells. J Biotechnol 155(2):225–231. https://doi.org/10.1016/j.jbiotec.2011.06.020
Chen F, Kou T, Fan L, Zhou Y, Ye Z, Zhao L, Tan W-S (2011) The combined effect of sodium butyrate and low culture temperature on the production, sialylation, and biological activity of an antibody produced in CHO cells. Biotechnol Bioprocess Eng 16(6):1157–1165. https://doi.org/10.1007/s12257-011-0069-8
Jiang Z, Sharfstein ST (2008) Sodium butyrate stimulates monoclonal antibody over-expression in CHO cells by improving gene accessibility. Biotechnol Bioeng 100(1):189–194. https://doi.org/10.1002/bit.21726
Mimura Y, Lund J, Church S, Dong S, Li J, Goodall M, Jefferis R (2001) Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. J Immunol Methods 247(1–2):205–216. https://doi.org/10.1016/S0022-1759(00)00308-2
Cherlet M, Marc A (2000) Stimulation of monoclonal antibody production of hybridoma cells by butyrate: evaluation of a feeding strategy and characterization of cell behaviour. Cytotechnology 32(1):17–29. https://doi.org/10.1023/A:1008069523163
Sung YH, Lee JS, Park SH, Koo J, Lee GM (2007) Influence of co-down-regulation of caspase-3 and caspase-7 by siRNAs on sodium butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin. Metab Eng 9(5–6):452–464. https://doi.org/10.1016/j.ymben.2007.08.001
Oh HK, So MK, Yang J, Yoon HC, Ahn JS, Lee JM, Kim JT, Yoo JU, Byun TH (2005) Effect of N-acetylcystein on butyrate-treated chinese hamster ovary cells to improve the production of recombinant human interferon-β-1a. Biotechnol Prog 21(4):1154–1164. https://doi.org/10.1021/bp050057v
Kim NS, Lee GM (2002) Inhibition of sodium butyrate-induced apoptosis in recombinant Chinese hamster ovary cells by constitutively expressing antisense RNA of caspase-3. Biotechnol Bioeng 78(2):217–228. https://doi.org/10.1002/bit.10191
Kim NS, Lee GM (2000) Overexpression of bcl-2 inhibits sodium butyrate-induced apoptosis in Chinese hamster ovary cells resulting in enhanced humanized antibody production. Biotechnol Bioeng 71 (3):184–193. https://doi.org/10.1002/1097-0290(2000)71:3<184::AID-BIT1008>3.0.CO;2-W
Sung YH, Hwang SF - Lee GM, Lee GM Influence of down-regulation of caspase-3 by siRNAs on sodium-butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin (1096–7176 (Print))
Inoue Y, Fujisawa M, Shoji M, Hashizume S, Katakura Y, Shirahata S (2000) Enhanced antibody production of human–human hybridomas by retinoic acid. Cytotechnology 33(1–3):83–88. https://doi.org/10.1023/A:1008155609072
Inoue Y, Fujisawa M, Kawamoto S, Shoji M, Hashizume S, Fujii M, Katakura Y, Shirahata S (1999) Effectiveness of vitamin A acetate for enhancing the production of lung cancer specific monoclonal antibodies. Cytotechnology 31(1–2):77–83. https://doi.org/10.1023/A:1008016020785
Amiri MM, Jeddi-Tehrani M, Kazemi T, Bahadori M, Maddah M, Hojjat-Farsangi M, Khoshnoodi J, Rabbani H, Shokri F (2013) Construction and characterization of a new chimeric antibody against HER2. Immunotherapy 5(7):703–715. https://doi.org/10.2217/imt.13.67
Tahmasebi F, Kazemi T, Amiri MM, Khoshnoodi J, Mahmoudian J, Bayat AA, Jeddi-Tehrani M, Rabbani H, Shokri F (2014) In vitro assessment of the effects of anti-HER2 monoclonal antibodies on proliferation of HER2-overexpressing breast cancer cells. Immunotherapy 6(1):(1750–7448 (Electronic)):43–49. https://doi.org/10.2217/imt.13.156
Kazemi T, Tahmasebi F, Bayat AA, Mohajer N, Khoshnoodi J, Jeddi-Tehrani M, Rabbani H, Shokri F (2011) Characterization of novel murine monoclonal antibodies directed against the extracellular domain of human HER2 tyrosine kinase receptor. Hybridoma 30(4):347–353. https://doi.org/10.1089/hyb.2011.0023
Hajighasemi FS-YA., Shokri F (2004) Measurement of affinity constant of anti-human IgG monoclonal antibodies by an ELISA-based method. Irn J Immunol 1(3):154–161
Golsaz Shirazi F, Mohammadi H, Fau-Amiri MM, Singethan K, Xia Y, Bayat AA, Bahadori M, Rabbani H, Jeddi-Tehrani M, Protzer U, Shokri F (2014) Monoclonal antibodies to various epitopes of hepatitis B surface antigen inhibit hepatitis B virus infection. J Gastroenterol Hepatol 29(5):1083–1091. https://doi.org/10.1111/jgh.12483
Du Z, Treiber D, McCarter JD, Fomina-Yadlin D, Saleem RA, McCoy RE, Zhang Y, Tharmalingam T, Leith M, Follstad BD, Dell B, Grisim B, Zupke C, Heath C, Morris AE, Reddy P (2015) Use of a small molecule cell cycle inhibitor to control cell growth and improve specific productivity and product quality of recombinant proteins in CHO cell cultures. Biotechnol Bioeng 112(1):141–155. https://doi.org/10.1002/bit.25332
Rahimpour A, Ahani R, Najaei A, Adeli A, Barkhordari F, Mahboudi F (2016) Development of genetically modified chinese hamster ovary host cells for the enhancement of recombinant tissue plasminogen activator expression. The Malaysian Journal of Medical Sciences: MJMS 23(2):6–13
Sharow KA, Temkin B, Asson-Batres MA (2012) Retinoic acid stability in stem cell cultures. Int J Dev Biol 56(4):273–278. https://doi.org/10.1387/ijdb.113378ks
Jefferis R (2016) Posttranslational Modifications and the Immunogenicity of Biotherapeutics. J Immunol Res 2016:5358272. https://doi.org/10.1155/2016/5358272
Acknowledgements
This work was supported partially by grants from Tarbiat Modares University and Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Rahimi-Zarchi, M., Shojaosadati, S.A., Amiri, M.M. et al. All-trans retinoic acid in combination with sodium butyrate enhances specific monoclonal antibody productivity in recombinant CHO cell line. Bioprocess Biosyst Eng 41, 961–971 (2018). https://doi.org/10.1007/s00449-018-1927-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1927-y