Abstract
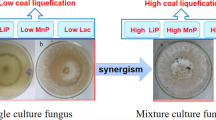
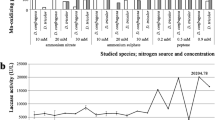
The lignin-modifying enzymes (LMEs) play an important role in decomposition of agricultural residues, which contain a certain amount of lignin. In this study, the production of LMEs by three co-cultivated combinations of Phlebia radiata, Dichomitus squalens and Ceriporiopsis subvermispora and the respective monocultures was comparatively investigated. Laccase and manganese peroxidases (MnP) were significantly promoted in the co-culture of P. radiata and D. squalens, and corncob was verified to be beneficial for laccase and MnP production. Moreover, laccase production by co-culture of P. radiata and D. squalens with high ratio of glucose to nitrogen was higher than low ratio under carbon- and nitrogen-meager conditions. New laccase isoenzymes measured by Native-PAGE were stimulated by co-cultured P. radiata with D. squalens or C. subvermispora, respectively, growing in the defined medium containing corncob, but the expression of laccase was greatly restrained by the co-culturing of D. squalens with C. subvermispora. This study showed that the synergistic and depressing effects of co-cultivation of P. radiata, D. squalens and C. subvermispora on LMEs were species specific.






Similar content being viewed by others
Abbreviations
- LMEs:
-
Lignin-modifying enzymes
- MnP:
-
Manganese peroxidases
- LiP:
-
Lignin peroxidase
- VP:
-
Versatile peroxidase
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt
- VA:
-
Veratryl alcohol
- PR:
-
Phlebia radiata
- DS:
-
Dichomitus squalens
- CS:
-
Ceriporiopsis subvermispora
- PDA:
-
Potato dextrose agar
- DNS:
-
3,5-Dinitrosalicylic acid
- Native-PAGE:
-
Native polyacrylamide gel electrophoresis
References
Roncero MB, Torres AL, Colom JF, Vidal T (2003) TCF bleaching of wheat straw pulp using ozone and xylanase. Part A: paper quality assessment. Bioresour Technol 87:305–314
Jeffries TW (1990) Biodegradation of lignin–carbohydrate complexes. Biodegrad 1:163–176
Kersten P, Cullen D (2007) Extracellular oxidative systems of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Fungal Genet Biol 44:77–87
Higuchi T (2006) Look back over the studies of lignin biochemistry. J Wood Sci 52:2–8
Geib SM, Filley TR, Hatcher PG, Hoover K, Carlson JE, Jimenez-Gasco MD, Nakagawa-Izumi A, Sleighter RL, Tien M (2008) Lignin degradation in wood-feeding insects. PNAS 105:12932–12937
Hammel KE, Cullen D (2008) Role of fungal peroxidases in biological ligninolysis. Curr Opin Plant Biol 11:349–355
Hatakka A (1994) Lignin-modifying enzymes from selected white rot fungi: production and role in lignin degradation. FEMS Microbiol Rev 13:125–135
Lundell TK, Mäkelä MR, Hildén K (2010) Lignin-modifying enzymes in filamentous basidiomycetes—ecological, functional and phylogenetic review. J Basic Microbiol 50:5–20
Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ (2006) Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 72:3593–3599
Hu HL, van den Brink J, Gruben BS, Wösten HAB, Gu JD, de Vries RP (2011) Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int Biodeterior Biodegrad 65:248–252
Chi YJ, Hatakka A, Maijala P (2007) Can co-culturing of two white-rot fungi increase lignin degradation and the production of lignin-degrading enzymes? Int Biodeterior Biodegrad 59:32–39
Chen QH, Sven K, Thomas H, Steffen R, Susanne Z (2011) Co-cultured production of lignin-modifying enzymes with white-rot fungi. Appl Biochem Biotechnol 165:700–718
Velazquez-Cedẽno MA, Farnet AM, Ferré E, Savoie JM (2004) Variations of lignocellulosic activities in dual cultures of Pleurotus ostreatus and Trichoderma longibrachiatum on unsterilized wheat straw. Mycologia 96:712–719
Zhang H, Hong YZ, Xiao YZ, Yuan J, Tu XM, Zhang XQ (2006) Efficient production of laccases by Trametes sp. AH28–2 in cocultivation with a Trichoderma strain. Appl Microbiol Biotechnol 73:89–94
Gutierrez-Correa M, Tengerdy RP (1997) Production of cellulase on sugarcane bagasse by fungal mixed culture solid substrate fermentation. Biotechnol Lett 19:665–667
Koroleva OV, Gavrilova VP, Stepanova EV, Lebedeva VI, Sverdlova NI, Landesman EO, Yavmetdinov IS, Yaropolov AI (2002) Production of lignin modifying enzymes by co-cultivated white-rot fungi Cerrena maxima and Coriolus hirsutus and characterization of laccase from Cerrena maxima. Enzym Microbiol Technol 30:573–580
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white-rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Kirk TK, Croan S, Tien M, Murtagh KE, Farrell RE (1986) Production of multiple ligninases by Phanerochaete chrysosporium: effect of selected growth conditions and use of a mutant strain. Enzym Microbiol Technol 8:27–32
Paszczynski A, Crawford RL, Huynh VB (1988) Manganese peroxidase of Phanerochaete chrysosporium: purification. In: Wood WA, Kellogg ST (eds) Methods in Enzymology. Academic Press, New York
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Dorado J, Almendros G, Camarero S, Martínez AT, Vares T, Hatakka A (1999) Transformation of wheat straw in the course of solid-state fermentation by four ligninolytic basidiomycetes. Enzym Microbiol Technol 25:605–612
Li A, Antizar-Ladislao B, Khraisheh M (2007) Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioprocess Biosyst Eng 30:189–196
Song C, Hu H, Zhu S, Wang G, Chen G (2004) Nonisothermal catalytic liquefaction of corn stalk in subcritical and supercritical water. Energy Fuels 18:90–96
Zhang ML, Fan YT, Xing Y, Pan CM, Zhang GS, Lay JJ (2007) Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenerg 31:250–254
Vaughan T, Seo CW, Marshall WE (2001) Removal of selected metal ions from aqueous solution using modified corncobs. Bioresour Technol 78:133–139
Howard RL, Abotsi E, Jansen van Rensburg EL, Howard S (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol 2:602–619
Sanchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46
Vares T, Kalsi M, Hatakka A (1995) Lignin peroxidases, manganese peroxidases, and other ligninolytic enzymes produced by Phlebia radiata during solid-state fermentation of wheat straw. Appl Environ Microbiol 61:3515–3520
Elisashvili V, Kachlishvili E (2009) Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J Biotehnol 144:37–42
Tekere M, Zvauya R, Read JS (2001) Ligninolytic enzyme production in selected sub-tropical white rot fungi under different culture conditions. J Basic Microbiol 41:115–129
Šušla M, Novotný C, Svobodová K (2007) The implication of Dichomitus squalens laccase isoenzymes in dye decolorization by immobilized fungal cultures. Bioresour Technol 98:2109–2115
Garcia TA, Santiago MF, Ulhoa CJ (2007) Studies on the Pycnoporus sanguineus CCT-4518 laccase purified by hydrophobic interaction chromatography. Appl Microbiol Biotechnol 75:311–318
Fonseca MI, Shimizu E, Zapata PD, Villalba LL (2010) Copper inducing effect on laccase production of white rot fungi native from Misiones (Argentina). Enzym Microbiol Technol 46:534–539
Acknowledgments
This study was financially supported by the Natural Science Foundation of Zhejiang Province, China (Y3090026).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Y.-C. Dong and W. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dong, YC., Wang, W., Hu, ZC. et al. The synergistic effect on production of lignin-modifying enzymes through submerged co-cultivation of Phlebia radiata, Dichomitus squalens and Ceriporiopsis subvermispora using agricultural residues. Bioprocess Biosyst Eng 35, 751–760 (2012). https://doi.org/10.1007/s00449-011-0655-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0655-3