Abstract
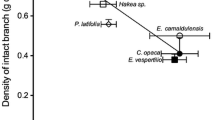
Xylem structure and function is proposed to reflect an evolutionary balance between demands for efficient movement of water to the leaf canopy and resistance to cavitation during high xylem tension. Water use efficiency (WUE) affects this balance by altering the water cost of photosynthesis. Therefore species of greater WUE, such as C4 plants, should have altered xylem properties. To evaluate this hypothesis, we assessed the hydraulic and anatomical properties of 19 C3 and C4 woody species from arid regions of the American west and central Asia. Specific conductivity of stem xylem (K s ) was 16%–98% lower in the C4 than C3 shrubs from the American west. In the Asian species, the C3 Nitraria schoberi had similar and Halimodendron halodendron higher K s values compared with three C4 species. Leaf specific conductivity (K L ; hydraulic conductivity per leaf area) was 60%–98% lower in the C4 than C3 species, demonstrating that the presence of the C4 pathway alters the relationship between leaf area and the ability of the xylem to transport water. C4 species produced similar or smaller vessels than the C3 shrubs except in Calligonum, and most C4 shrubs exhibited higher wood densities than the C3 species. Together, smaller conduit size and higher wood density indicate that in most cases, the C4 shrubs exploited higher WUE by altering xylem structure to enhance safety from cavitation. In a minority of cases, the C4 shrubs maintained similar xylem properties but enhanced the canopy area per branch. By establishing a link between C4 photosynthesis and xylem structure, this study indicates that other phenomena that affect WUE, such as atmospheric CO2 variation, may also affect the evolution of wood structure and function.





Similar content being viewed by others
References
Baker JT, Allen LH Jr, Boote KL, Jones P, Jones JW (1990) Rice photosynthesis and evapotransiration in subambient, ambient, and superambient carbon dioxide concentrations. Agron J 82:834–840
Berner RA, Kothavala Z (2001) GEOCARB III: a revised model of atmospheric CO2 over phanerozoic time. Am J Sci 301:182–204
Black CJ (1973) Photosynthetic carbon fixation in relation to net CO2 uptake. Annu Rev Plant Physiol 24:253–286
Caldwell MM, White RS, Moore RT, Camp LB (1977) Carbon balance, productivity, and water use of cold-winter desert shrub communities dominated by C3 and C4 species. Oecologia 29:275–300
Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC (2002) Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. Am J Bot 89:820–828
Dettori ML, Balliette JF, Young JA, Evans RA (1984) Temperature profiles for germination of two species of winterfat. J Range Manage 37:218–222
Dryden P, Van Alfen NK (1983) Use of the pressure bomb for hydraulic conductance studies. J Exp Bot 34:523–528
Hacke UG, Sperry JS (2001) Functional and ecological xylem anatomy. Perspect Plant Ecol Evol Syst 4:97–115
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloch KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Hubbard RM, Bond BJ, Ryan MG (1999) Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol 19:165–172
Kocacinar F (2004) Photosynthetic pathway and hydraulic architecture in higher plants. PhD Thesis, University of Toronto, Toronto
Kocacinar F, Sage RF (2003) Photosynthetic pathway alters xylem structure and hydraulic function in herbaceous plants. Plant Cell Environ 26:2015-2026
Larcher W (1995) Physiological plant ecology, 3rd edn. Springer, Berlin Heidelberg New York
Ludlow MM (1976) Ecophysiology of C4 grasses. In: Lange OL, Kappen L, Schulze E-D (eds) Water and plant life: problems and modern approaches, vol 19. Springer, Berlin Heidelberg New York, pp 364–386
Martínez-Vilalta J, Prat E, Oliveras I, Piñol J (2002) Xylem hydraulic properties of roots and stems of nine Mediterranean woody species. Oecologia 133:19–29
Osmond CB, Björkman O, Anderson DJ (1980) Physiological processes in plant ecology: toward a synthesis with Atriplex. Springer, Berlin Heidelberg New York
Pallardy SG, Cermak J, Ewers FW, Kaufmann MR, Parker WC, Sperry JS (1995) Water transport dynamics in trees and stands. In: Smith WK, Hinckley TM (eds) Resource physiology of conifers: acquisition, allocation, and utilization. Academic Press, San Diego, pp 301–375
Pearcy RW, Ehleringer J (1984) Comparative ecophysiology of C3 and C4 plants. Plant Cell Environ 7:1–13
Pockman WT, Sperry JS (2000) Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am J Bot 87:1287–1299
Potter JR, Jones JW (1977) Leaf area partitioning as an important factor in growth. Plant Physiol 59:10–14
Schulze E-D, Hall AE (1982) Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopedia of plant physiology: physiological plant ecology. II. Water relations and carbon assimilation, vol II. Springer, Berlin Heidelberg New York, pp 181–230
Schulze ED, Hall AE, Lange OL, Evenari M, Kappen L, Buschbom U (1980) Long-term effects of drought on wild and cultivated plants in the Negev Desert.1. Maximal rates of net photosynthesis. Oecologia 45:11–18
Sobrado MA (2000) Relation of water transport to leaf gas exchange properties in three mangrove species. Trees 14:258–262
Sperry JS, Hacke UG (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Funct Ecol 16:367–378
Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE (1994) Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 75:1736–1752
Tyree MT, Sperry JS (1989) Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol 40:19–38
Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction. IAWA J 15:335–360
Walter H, Box EO (1983) Middle Asian deserts. In: West NE (ed) Ecosystems of the world 5: temperate deserts and semi-deserts. Elsevier, Amsterdam, pp 79–104
Wheeler EA, Baas P (1991) A survey of the fossil record for dicotyledonous wood and its significance for evolutionary and ecological wood anatomy. IAWA Bull 12:275–332
Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292:686–693
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer, Berlin Heidelberg New York
Zimmermann MH, Jeje AA (1981) Vessel-length distribution in stems of some American woody plants. Can J Bot 59:1882–1892
Zotz G, Tyree MT, Cochard H (1994) Hydraulic architecture, water relations and vulnerability to cavitation of Clusia uvitana Pittier: a C3-CAM tropical hemiepiphyte. New Phytol 127:287–295
Acknowledgements
The authors wish to thank Tammy Sage, Thomas Fung, Kathy Sault, and Mathew Lam for their help in the study and Nancy Dengler and Spencer Barrett for helpful discussions. This research was supported by a scholarship from the Ministry of Education of Turkey to F.K. and by NSERC grant #OGP0154273 to R.F.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kocacinar, F., Sage, R.F. Photosynthetic pathway alters hydraulic structure and function in woody plants. Oecologia 139, 214–223 (2004). https://doi.org/10.1007/s00442-004-1517-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1517-3