Abstract
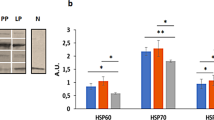
Currently, no reports exist concerning the expression patterns and developmental changes of heat shock proteins (HSPs) in the reproductive system of the male rabbit. In the present study, the testes of rabbits were collected at post-natal months 1, 2, 3, 4, 5, and 40. HSP60, HSC70, HSP90, and HSPA2 were detected by both Western blot and immunohistochemical methods. The expression levels of HSP60 and HSC70 showed no apparent change during the developmental progress. HSP90 increased at the second month; prior to the third month, HSPA2 was expressed at a low level. Immunohistochemistry localized HSP60 in the cytoplasm of all of the cell types in the testis and in the apical pole of the spermatids. The distribution pattern of HSC70 and HSP90 was similar, both being mainly located in the spermatids of stage VII-VIII and in the cytoplasm of the spermatogonium. HSPA2 staining was mainly observed in the cytoplasm of pachytene spermatocytes and spermatids in testes of 3-, 4-, 5-, and 40-month-old rabbits. These results provide a basic reference point for studying the functions of HSPs in the male rabbit reproductive system and should be beneficial for the future determination of the mechanisms of heat shock on male rabbit fertility.







Similar content being viewed by others
References
Allen RL, O'Brien DA, Eddy EM (1988) A novel hsp70-like protein (P70) is present in mouse spermatogenetic cells. Mol Cell Biol 8:828–832
Allen JW, Collins BW, Merrick BA, He C, Selkirk JK, Poorman-Allen P, Dresser ME, Eddy EM (1996) HSP70-2 is part of the synaptonemal complex in mouse and hamster spermatocytes. Chromosoma 104:414–421
Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ (2004) Tyrosine phosphorylation activates surface chaperones facilitating sperm–zona recognition. J Cell Sci 117:3645–3657
Craig EA, Weissman JS, Horwich AL (1994) Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell 78:365–372
Dix DJ (1997) Hsp70 expression and function during gametogenesis. Cell Stress Chaperones 2:73–77
Dix DJ, Rosario-Herrle M, Gotoh H, Mori C, Goulding EH, Barrett CV, Eddy EM (1996a) Developmentally regulated expression of Hsp70-2 and a Hsp70-2/lacZ transgene during spermatogenesis. Dev Biol 174:310–321
Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM (1996b) Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci USA 93:3264–3268
Ecroyd H, Jones RC, Aitken RJ (2003) Tyrosine phosphorylation of HSP-90 during mammalian sperm capacitation. Biol Reprod 69:1801–1807
Gondos B, Renston RH, Conner LA (1973) Ultrastructure of germ cells and Sertoli cells in the postnatal rabbit testis. Am J Anat 136:427–439
Gruppi CM, Wolgemuth DJ (1993) HSP86 and HSP84 exhibit cellular specificity of expression and co-precipitate with an HSP70 family member in the murine testis. Dev Genet 14:119–126
Gruppi CM, Zakeri ZF, Wolgemuth DJ (1991) Stage and lineage-regulated expression of two hsp90 transcripts during mouse germ cell differentiation and embryogenesis. Mol Reprod Dev 28:209–217
Hickey E, Brandon SE, Smale G, Lloyd D, Weber LA (1989) Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol Cell Biol 9:2615–2626
Huang SY, Tam MF, Hsu YT, Lin JH, Chen HH, Chuang CK, Chen MY, King YT, Lee WC (2005) Developmental changes of heat-shock proteins in porcine testis by a proteomic analysis. Theriogenology 64:1940–1955
Kamaruddin M, Kroetsch T, Basrur PK, Hansen PJ, King WA (2004) Immunolocalization of heat shock protein 70 in bovine spermatozoa. Andrologia 36:327–334
Lee SJ (1990) Expression of HSP86 in male germ cells. Mol Cell Biol 10:3239–3242
Lindquist S (1986) The heat shock response. Annu Rev Biochem 55:1151–1191
Matwee C, Kamaruddin M, Betts DH, Basrur PK, King A (2001) The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod 7:829–837
Meinhardt A, Parvinen M, Bacher M, Aumüller G, Hakovirta H, Yagi A, Seitz J (1995) Expression of mitochondrial heat shock protein 60 in distinct cell types and defined stages of rat seminiferous epithelium. Biol Reprod 52:798–807
Minami Y, Kawasaki H, Miyata Y, Suzuki K, Yahara I (1991) Analysis of native forms and isoform compositions of the mouse 90-kDa heat shock protein, HSP90. J Biol Chem 266:10099–10103
Moore SK, Kozak C, Robinson EA, Ullrich SJ, Appella E (1989) Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem 264:5343–5351
Morton D (1988) The use of rabbits in male reproductive toxicology. Environ Health Perspect 77:5–9
Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS (2000) The role of heat shock proteins in reproduction. Hum Reprod Update 6:149–159
Ohsako S, Bunick D, Hayashi Y (1995) Immunocytochemical observation of the 90 kD heat shock protein (HSP90): high expression in primordial and pre-meiotic germ cells of male and female rat gonads. J Histochem Cytochem 43:67–76
Paranko J, Seitz J, Meinhardt A (1996) Developmental expression of heat shock protein 60 (HSP60) in the rat testis and ovary. Differentiation 60:159–167
Raab LS, Polakoski KL, Hancock LW, Hamilton DW (1995) Characterization of the heat shock protein P70 in rat spermatogenic cells. Mol Reprod Dev 40:186–195
Ricken AM, Viebahn C (2002) Stage-specific expression of the mitochondrial germ cell epitope PG2 during postnatal differentiation of rabbit germ cells. Biol Reprod 67:196–203
Sarge KD, Cullen KE (1997) Regulation of hsp expression during rodent spermatogenesis. Cell Mol Life Sci 53:191–197
Son WY, Hwang SH, Han CT, Lee JH, Kim S, Kim YC (1999) Specific expression of heat shock protein HspA2 in human male germ cells. Mol Hum Reprod 5:1122–1126
Son WY, Han CT, Hwang SH, Lee JH, Kim S, Kim YC (2000) Repression of hspA2 messenger RNA in human testis with abnormal spermatogenesis. Fertil Steril 73:1138–1144
Spinaci M, Volpe S, Bernardini C, De Ambrogi M, Tamanini C, Seren E, Galeati G (2005) Immunolocalization of heat shock protein 70 (Hsp70) in boar spermatozoa and its role during fertilization. Mol Reprod Dev 72:534–541
Volpe S, Galeati G, Bernardini C, Tamanini C, Mari G, Zambelli D, Seren E, Spinaci M (2008) Comparative immunolocalization of heat shock proteins (Hsp)-60, -70, -90 in boar, stallion, dog and cat spermatozoa. Reprod Domest Anim 43:385–392
Walsh A, Whelan D, Bielanowicz A, Skinner B, Aitken RJ, O'Bryan MK, Nixon B (2008) Identification of the molecular chaperone, heat shock protein 1 (chaperonin 10), in the reproductive tract and in capacitating spermatozoa in the male mouse. Biol Reprod 78:983–993
Welch YJ (1992) Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 72:1063–1080
Werner A, Meinhardt A, Seitz J, Bergmann M (1997) Distribution of heat-shock protein 60 immunoreactivity in testes of infertile men. Cell Tissue Res 288:539–544
Wu Y, Luo H, Liu J, Kang D, McNeilly AS, Cui S (2010) LIM homeodomain transcription factor Isl-1 enhances follicle stimulating hormone-β and luteinizing hormone-β gene expression and mediates the activation of leptin on gonadotropin synthesis. Endocrinology 151:4787–4800
Author information
Authors and Affiliations
Corresponding author
Additional information
Yingjie Wu and Yangli Pei contributed equally to this work.
This work was supported by the Natural Science Foundation of China (31001009) and Earmarked Fund for Modern Agro-industry Technology Research System (nycytx-44).
Rights and permissions
About this article
Cite this article
Wu, Y., Pei, Y. & Qin, Y. Developmental expression of heat shock proteins 60, 70, 90, and A2 in rabbit testis. Cell Tissue Res 344, 355–363 (2011). https://doi.org/10.1007/s00441-011-1151-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1151-4