Abstract
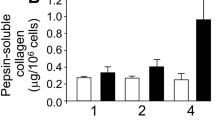
Modulation of the actin cytoskeleton in chondrocytes has been used to prevent or reverse dedifferentiation and to enhance protein synthesis. We have hypothesized that an actin-modifying agent, staurosporine, could be used with fibrochondrocytes to increase the gene expression and synthesis of critical fibrocartilage proteins. A range of concentrations (0.1–100 nM) was applied to fibrochondrocytes in monolayer and evaluated after 24 h and after 4 days. High-dose staurosporine treatment (10–100 nM) increased cartilage oligomeric matrix protein 60– to 500-fold and aggrecan gene expression two-fold. This effective range of staurosporine was then applied to scaffoldless tissue-engineered fibrochondrocyte constructs for 4 weeks. Whereas glycosaminoglycan synthesis was not affected, collagen content doubled, from 27.6 ± 8.8 μg in the untreated constructs to 55.2 ± 12.2 μg per construct with 100 nM treatment. When analyzed for specific collagens, the 10-nM group showed a significant increase in collagen type I content, whereas collagen type II was unaffected. A concomitant dose-dependent reduction was noted in construct contraction, reflecting the actin-disrupting action of staurosporine. Thus, staurosporine increases the gene expression for important matrix proteins and can be used to enhance matrix production and reduce contraction in tissue-engineered fibrocartilage constructs.





Similar content being viewed by others
References
Benya PD (1988) Modulation and reexpression of the chondrocyte phenotype; mediation by cell shape and microfilament modification. Pathol Immunopathol Res 7:51–54
Benya PD, Padilla SR (1986) Modulation of the rabbit chondrocyte phenotype by retinoic acid terminates type II collagen synthesis without inducing type I collagen: the modulated phenotype differs from that produced by subculture. Dev Biol 118:296–305
Borge L, Lemare F, Demignot S, Adolphe M (1997) Restoration of the differentiated functions of serially passaged chondrocytes using staurosporine. In Vitro Cell Dev Biol Anim 33:703–709
Darling EM, Athanasiou KA (2005) Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res 23:425–432
Deschner J, Rath-Deschner B, Agarwal S (2005a) Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthritis Cartilage 14:264–272
Deschner J, Wypasek E, Ferretti M, Rath B, Anghelina M, Agarwal S (2005b) Regulation of RANKL by biomechanical loading in fibrochondrocytes of meniscus. J Biomech 39:1796–1803
Diaz-Romero J, Gaillard JP, Grogan SP, Nesic D, Trub T, Mainil-Varlet P (2005) Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol 202:731–742
Fink C, Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Guilak F (2001) The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis Cartilage 9:481–487
Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane crosstalk between the extracellular matrix—cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2:793–805
Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R (2007) The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials 28:4068–4077
Gunja NJ, Athanasiou K (2007) Passage and reversal effects on gene expression of bovine meniscal fibrochondrocytes. Arthritis Res Ther 9:R93
Hidaka C, Ibarra C, Hannafin JA, Torzilli PA, Quitoriano M, Jen SS, Warren RF, Crystal RG (2002) Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering. Tissue Eng 8:93–105
Hoben G, Hu J, James R, Athanasiou KA (2007) Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng 13:939–946
Honda MJ, Yada T, Ueda M, Kimata K (2004) Cartilage formation by serial passaged cultured chondrocytes in a new scaffold: hybrid 75:25 poly(L-lactide-epsilon-caprolactone) sponge. J Oral Maxillofac Surg 62:1510–1516
Hu JC, Athanasiou KA (2006) A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12:969–979
Imler SM, Doshi AN, Levenston ME (2004) Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage 12:736–744
Isoda K, Saito S (1998) In vitro and in vivo fibrochondrocyte growth behavior in fibrin gel: an immunohistochemical study in the rabbit. Am J Knee Surg 11:209–216
Kambic HE, Futani H, McDevitt CA (2000) Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen 8:554–561
Kaps C, Frauenschuh S, Endres M, Ringe J, Haisch A, Lauber J, Buer J, Krenn V, Haupl T, Burmester GR, Sittinger M (2006) Gene expression profiling of human articular cartilage grafts generated by tissue engineering. Biomaterials 27:3617–3630
Kinner B, Spector M (2001) Smooth muscle actin expression by human articular chondrocytes and their contraction of a collagen-glycosaminoglycan matrix in vitro. J Orthop Res 19:233–241
Lee CR, Grodzinsky AJ, Spector M (2003) Modulation of the contractile and biosynthetic activity of chondrocytes seeded in collagen-glycosaminoglycan matrices. Tissue Eng 9:27–36
Lietman SA, Hobbs W, Inoue N, Reddi AH (2003) Effects of selected growth factors on porcine meniscus in chemically defined medium. Orthopedics 26:799–803
Loty S, Foll C, Forest N, Sautier JM (2000) Association of enhanced expression of gap junctions with in vitro chondrogenic differentiation of rat nasal septal cartilage-released cells following their dedifferentiation and redifferentiation. Arch Oral Biol 45:843–856
Mobley PL, Hedberg K, Bonin L, Chen B, Griffith OH (1994) Decreased phosphorylation of four 20-kDa proteins precedes staurosporine-induced disruption of the actin/myosin cytoskeleton in rat astrocytes. Exp Cell Res 214:55–66
Mueller SM, Schneider TO, Shortkroff S, Breinan HA, Spector M (1999a) Alpha-smooth muscle actin and contractile behavior of bovine meniscus cells seeded in type I and type II collagen-GAG matrices. J Biomed Mater Res 45:157–166
Mueller SM, Shortkroff S, Schneider TO, Breinan HA, Yannas IV, Spector M (1999b) Meniscus cells seeded in type I and type II collagen-GAG matrices in vitro. Biomaterials 20:701–709
Mukherjee P, Rachita C, Aisen PS, Pasinetti GM (2001) Non-steroidal anti-inflammatory drugs protect against chondrocyte apoptotic death. Clin Exp Rheumatol 19:S7–11
Newman P, Watt FM (1988) Influence of cytochalasin D-induced changes in cell shape on proteoglycan synthesis by cultured articular chondrocytes. Exp Cell Res 178:199–210
Pangborn CA, Athanasiou KA (2005a) Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng 11:1141–1148
Pangborn CA, Athanasiou KA (2005b) Growth factors and fibrochondrocytes in scaffolds. J Orthop Res 23:1184–1190
Perez LM, Milkiewicz P, Ahmed-Choudhury J, Elias E, Ochoa JE, Sanchez Pozzi EJ, Coleman R, Roma MG (2006) Oxidative stress induces actin-cytoskeletal and tight-junctional alterations in hepatocytes by a Ca2+-dependent, PKC-mediated mechanism: protective effect of PKA. Free Radic Biol Med 40:2005–2017
Pirttiniemi P, Kantomaa T (1998) Effect of cytochalasin D on articular cartilage cell phenotype and shape in long-term organ culture. Eur J Orthod 20:491–499
Reddy GK, Enwemeka CS (1996) A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 29:225–229
Rosenberg K, Olsson H, Morgelin M, Heinegard D (1998) Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem 273:20397–20403
Sakurada K, Seto M, Sasaki Y (1998) Dynamics of myosin light chain phosphorylation at Ser19 and Thr18/Ser19 in smooth muscle cells in culture. Am J Physiol 274:C1563–C1572
Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, Schlegel J (2002) Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage 10:62–70
Shieh AC, Athanasiou KA (2007) Dynamic compression of single cells. Osteoarthritis Cartilage 15:328–334
Tanaka T, Fujii K, Kumagae Y (1999) Comparison of biochemical characteristics of cultured fibrochondrocytes isolated from the inner and outer regions of human meniscus. Knee Surg Sports Traumatol Arthrosc 7:75–80
Thur J, Rosenberg K, Nitsche DP, Pihlajamaa T, Ala-Kokko L, Heinegard D, Paulsson M, Maurer P (2001) Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem 276:6083–6092
Vanderploeg EJ, Imler SM, Brodkin KR, Garcia AJ, Levenston ME (2004) Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech 37:1941–1952
Verdonk PC, Forsyth RG, Wang J, Almqvist KF, Verdonk R, Veys EM, Verbruggen G (2005) Characterisation of human knee meniscus cell phenotype. Osteoarthritis Cartilage 13:548–560
Wakatsuki T, Wysolmerski RB, Elson EL (2003) Mechanics of cell spreading: role of myosin II. J Cell Sci 116:1617–1625
Zaleskas JM, Kinner B, Freyman TM, Yannas IV, Gibson LJ, Spector M (2004) Contractile forces generated by articular chondrocytes in collagen-glycosaminoglycan matrices. Biomaterials 25:1299–1308
Zaucke F, Dinser R, Maurer P, Paulsson M (2001) Cartilage oligomeric matrix protein (COMP) and collagen IX are sensitive markers for the differentiation state of articular primary chondrocytes. Biochem J 358:17–24
Zhang Z, Messana J, Hwang NS, Elisseeff JH (2006) Reorganization of actin filaments enhances chondrogenic differentiation of cells derived from murine embryonic stem cells. Biochem Biophys Res Commun 348:421–427
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors gratefully acknowledge NIAMS R01 AR 47839–2 for funding this work, and the Hertz Foundation for their support of G. Hoben.
Rights and permissions
About this article
Cite this article
Hoben, G.M., Athanasiou, K.A. Use of staurosporine, an actin-modifying agent, to enhance fibrochondrocyte matrix gene expression and synthesis. Cell Tissue Res 334, 469–476 (2008). https://doi.org/10.1007/s00441-008-0705-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-008-0705-6