Abstract
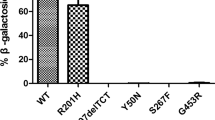
We report the modulating action of the L436F new polymorphism identified in the GLB1 gene of a patient affected by GM1 gangliosidosis with onset at 17 months and rapidly progressive psychomotor deterioration. Sequencing analysis and familial restriction studies revealed that the maternal allele of this patient carried the L436F polymorphism in cis with the known R201C mutation. The new mutation R68W was identified in his paternal allele. Since the GLB1 activity of the patient's leukocytes was very low and compatible with both the type-I and the type-II form of the disease, the potential impact of each mutation was investigated by expression studies in COS1 cells, and Western blots. Expression study of the R68W mutated allele resulted in no GLB1 activity. Transfection with a vector carrying the R201C mutation gave rise to a residual GLB1 activity, which, interestingly, was severely reduced in transfection with the L436F/R201C allele. These expression studies, together with co-transfection experiments, suggest that the R201C/L436F GLB1 "complex allele" leads to this patient's clinical and biochemical findings. The type-II phenotype of the disease is subdivided into late infantile and juvenile forms. The clinical and molecular characterization of this patient as late-infantile GM1 gangliosidosis is in keeping with a clear-cut division between the two sub forms of the type-II phenotype. The modulating role of the L436F polymorphism should be stressed as a cause of this patient's condition. This model suggests that the combination of missense mutations or polymorphisms should be evaluated when diagnosing inherited genetic disorders.


Similar content being viewed by others
References
Bonten EJ, van der Spoel A, Fornerod M, Grosveld G, d'Azzo A (1996) Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev 10:3156–3169
Boustany RM, Quian WH, Suzuki K (1993) Mutations in acid ß-galactosidase cause GM1-gangliosidosis in American Patients. Am J Hum Genet 53:881–888
Chakraborty S, Rafi MA, Wenger DA (1994) Mutations in the Lysosomal ß-galactosidase gene that cause the adult form of GM1-gangliosidosis. Am J Hum Genet 54:1004–1013
den Dunnen JT, Antonarakis SE (2001) Nomenclature for the description of human sequence variations. Hum Genet 109:121–124
Galjaard H (1980) Genetic metabolic diseases early diagnosis and prenatal analysis. Elsevier/North Holland Biochemical Press, Amsterdam, pp 825
Hara Y, Nishimoto J, Suzuki K (1994) Effects of double amino-acid substitution polymorphism in acid beta-galactosidase gene in two inbred strains of mice. Biochim Biophys Acta 1217:49–53
Hinek A (1996) Biological roles of the non-integrin elastin/laminin receptor. Biol Chem 377:471–480
Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J (1993) The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest 91:1198–1205
Hinek A, Wilson SE (2000) Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol 156:925–938
Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R (2000a) Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am J Hum Genet 66:859–872
Hinek A, Zhang S, Smith AC, Callahan JW (2000b) Impaired elastic-fiber assembly by fibroblasts from patients with either Morquio B disease or infantile GM1-gangliosidosis is linked to deficiency in the 67-kD spliced variant of beta-galactosidase. Am J Hum Genet 67:4–7
Kaye E, Shalish C, Livermore J, Taylor HA, Stevenson R, Breakefield O (1997) β-Galactosidase gene mutations in patient with slowly progressive GM1-gangliosidosis. J Child Neurol 12:242–247
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol method. J Biol Chem 193:265
Mecham RP, Hinek A, Entwistle R, Wrenn DS, Griffin GL, Senior RM (1989) Elastin binds to a multifunctional 67-kilodalton peripheral membrane protein. Biochemistry 28:3716–3722
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Mochizuki S, Brassart B, Hinek A (2002) Signaling pathways transduced through elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem 277:44854–44863
Morreau H, Galjart NJ, Gillemans N, Willemsen R, van der Horst GTJ, d'Azzo A (1989) Alternative splicing of β-galactosidase mRNA generates the classic lysosomal enzyme and a β-galactosidase-related protein. J Biol Chem 264:20655–20663
Morreau H, Bonten E, Zhou XY, d'Azzo A (1991) Organization of the gene encoding human lysosomal β-galactosidase. DNA Cell Biol 10:495–504
Morrone A, Bardelli T, Donati MA, Giorgi M, Di Rocco M, Gatti R, Parini R, Ricci R, Taddeucci G, D'Azzo A, Zammarchi E (2000) Beta-galactosidase gene mutations affecting the lysosomal enzyme and the elastin-binding protein in GM1-gangliosidosis patients with cardiac involvement. Hum Mutat 15:354–366
Nishimoto JE, Namba E, Okada S, Suzuki K (1991) Gm1-gangliosidosis (genetic beta-galactosidase deficiency): identification of four mutations in different clinical phenotypes among Japanese patients. Am J Hum Genet 49:566–574
Okamura-Oho Y, Zhang SQ, Hilson W, Hinek A, Callahan JW (1996) Early proteolytic cleavage with loss of a C-terminal fragment underlies altered processing of the β-galactosidase precursor in galactosialidosis. Biochem J 313:787–794
Oshima A, Tsuji A, Nagao Y, Sakuraba H, Suzuky Y (1988) Cloning, sequencing, and expression of cDNA for human β-galactosidase. Biochem Biophys Res Commun 157:238–244
Oshima A, Yoshida K, Itoh K, Kase R, Sakuraba H, Suzuki Y (1994) Intracellular processing and maturation of mutant gene products in hereditary β-galactosidase deficiency (β-galactosidosis). Hum Genet 93:109–114
Paschke E, Milos I, Kreimer-Erlacher H, Hoefler G, Beck M, Hoeltzenbein M, Kleijer W, Levade T, Michelakakis H, Radeva B (2001) Mutation analyses in 17 patients with deficiency in acid beta-galactosidase: three novel point mutations and high correlation of mutation W273L with Morquio disease type B. Hum Genet 109:159–166
Povey S, Lovering R, Bruford E, Wright M, Lush M, Wain H (2001) The HUGO Gene Nomenclature Committee (HGNC). Hum Genet 109:678–680
Privitera S, Prody CA, Callhan JW, Hinek A (1998) The 67 kDa enzymatically inactive alternatively spliced variant of β-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem 273:6319–6326
Pshezhetsky AV, Ashmarina M (2001) Lysosomal multienzyme complex: biochemistry, genetics, and molecular pathophysiology. Prog Nucleic Acid Res Mol Biol 69:81–114
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Laboratory Press, New York, section 16.42–16.44
Suzuki Y, Oshima A, Namba E (2001) ß-galactosidase deficiency (β-galactosidosis): GM1-gangliosidosis and Morquio B disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 3775–3809
Van der Spoel A, Bonten E, D'Azzo A (2000) Processing of lysosomal β-galactosidase. J Biol Chem 275:10035–100040
Van Dongen JM, Willemsen R, Ginns EI, Sips HJ, Tager JM, Barranger JA, Reuser AJ (1985) The subcellular localization of soluble and membrane-bound lysosomal enzymes in I-cell fibroblast: a comparative immunocytochemical study. Eur J Cell Biol 39:179–189
Yamamoto Y, Hake CA, Martin BM, Kretz KA, Ahernrindell AJ, Naylor SL, Mudd M, O'Brien JS (1990) Isolation, characterization and mapping of human acid beta-galactosidase cDNA. DNA Cell Biol 9:19–27
Yoshida K, Oshima A, Shimmoto M, Fukuhara Y, Sakuraba H, Yanagisawa N, Suzuki Y (1991) Human beta-galactosidase gene mutations in GM1-gangliosidosis: a common mutation among Japanese adult/chronic cases. Am J Hum Genet 49:435–442
Zhang S, McCarter JD, Okamura-Oho Y, Yaghi F, Hinek A, Withers SG, Callahan JW (1994) Kinetic mechanism and characterization of human b-galactosidase precursor secreted by permanently transfected Chinese hamster ovary cells. Biochem J 304:281–288
Acknowledgements
This work was partially supported by grants: Fondi Ateneo (MURST ex 60%) Azienda Ospedaliera Meyer, Association AMMEC and MPS Italy, NIH grants DK/NS52025 and GM60950 and Y. Campos by the Assisi Foundation of Memphis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caciotti, A., Bardelli, T., Cunningham, J. et al. Modulating action of the new polymorphism L436F detected in the GLB1 gene of a type-II GM1 gangliosidosis patient. Hum Genet 113, 44–50 (2003). https://doi.org/10.1007/s00439-003-0930-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-003-0930-8