Abstract
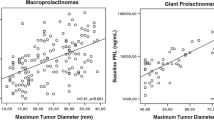
Prolactinoma is a rare pituitary adenoma secreting prolactin. Studies on diagnostics, treatment, and prognosis in pediatric prolactinoma patients are rare. We analyzed clinical presentation, response to treatment, and prognosis of 27 pediatric prolactinoma patients (10 m/17 f. based on patients’ records. Tumors included 6 microadenomas (tumor volume: median 0.2 cm3, range 0.01–0.4 cm3; serum prolactin at diagnosis: median 101 ng/ml, range 33–177 ng/ml), 15 macroadenomas (volume: median 3.3 cm3, range 0.4–25.8 cm3; prolactin: median 890 ng/ml, range 87–8624), and 3 giant adenomas (volume: median 44.5 cm3, range 38.6–93.5 cm3; prolactin: median 4720 ng/ml, range 317–10,400); data for 3 patients were not available. Dopamine agonist treatment (n = 22) was safe and effective, leading to reductions in tumor size (p < 0.01) and prolactin levels (p < 0.01). Threat to vision was the indication for decompressing surgery in three of seven operated patients. No patient was irradiated. Long-term functional capacity was not impaired when compared with other sellar masses (n = 235).
Conclusion: In pediatric prolactinoma, diagnosis is based on hyperprolactinemia and imaging. Dopamine agonist treatment is effective and safe. Overall survival and functional capacity as a measure of quality of survival were not impaired, indicating an optimistic prognosis. Surgery should be considered only in emergency situations of threatened visual function, not presenting a fast response to dopamine agonist treatment. Severe side effects of medication and lack of efficacy should be considered as contraindications.
What is Known: • In pediatric prolactinoma—a very rare pediatric neuroendocrinological disease—gender-related differences in terms of clinical presentation at initial diagnosis are known. • Due to the rareness of the disease, reports on long-term outcome and prognosis after childhood-onset prolactinoma based on prospective follow-up are not published. |
What is New: • Dopamine agonist treatment is efficient and safe for tumor volume reduction in pediatric prolactinoma and surgical interventions are recommended only for decompression of the optic chiasm in case of threat to vision. In case of inefficient response to medication, side effects or parental refuse, alternative therapeutic options should be considered. • Quality of life in terms of survival and functional capacity was not impaired in pediatric prolactinoma patients when compared with 235 long-term survivors of different sellar masses. |


Similar content being viewed by others
Abbreviations
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- FMH:
-
Fertigkeitenskala-Münster-Heidelberg
- OS:
-
Overall survival
References
Acharya SV, Gopal RA, Bandgar TR, Joshi SR, Menon PS, Shah NS (2009) Clinical profile and long term follow up of children and adolescents with prolactinomas. Pituitary 12:186–189
Atmaca M, Korkmaz S, Ustundag B, Ozkan Y (2015) Increased serum prolactin in borderline personality disorder. Int J Psychiatry Med 49:169–175
Catli G, Abaci A, Altincik A, Demir K, Can S, Buyukgebiz A, Bober E (2012) Hyperprolactinemia in children: clinical features and long-term results. J Pediatr Endocrinol Metab 25:1123–1128
Ciccarelli A, Daly AF, Beckers A (2005) The epidemiology of prolactinomas. Pituitary 8:3–6
Cohen-Inbar O, Xu Z, Schlesinger D, Vance ML, Sheehan JP (2015) Gamma knife radiosurgery for medically and surgically refractory prolactinomas: long-term results. Pituitary 18:820–830
Colao A, Di Sarno A, Landi ML, Scavuzzo F, Cappabianca P, Pivonello R, Volpe R, Di Salle F, Cirillo S, Annunziato L, Lombardi G (2000) Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patients. J Clin Endocrinol Metab 85:2247–2252
Colao A, Loche S, Cappa M, Di Sarno A, Landi ML, Sarnacchiaro F, Facciolli G, Lombardi G (1998) Prolactinomas in children and adolescents. Clinical presentation and long-term follow-up. J Clin Endocrinol Metab 83:2777–2780
Colao A, Vitale G, Cappabianca P, Briganti F, Ciccarelli A, De Rosa M, Zarrilli S, Lombardi G (2004) Outcome of cabergoline treatment in men with prolactinoma: effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysis. J Clin Endocrinol Metab 89:1704–1711
Dekkers OM, Lagro J, Burman P, Jorgensen JO, Romijn JA, Pereira AM (2010) Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: systematic review and meta-analysis. J Clin Endocrinol Metab 95:43–51
Eren E, Yapici S, Cakir ED, Ceylan LA, Saglam H, Tarim O (2011) Clinical course of hyperprolactinemia in children and adolescents: a review of 21 cases. J Clin Res Pediatr Endocrinol 3:65–69
Fideleff HL, Boquete HR, Suarez MG, Azaretzky M (2009) Prolactinoma in children and adolescents. Horm Res 72:197–205
Guaraldi F, Storr HL, Ghizzoni L, Ghigo E, Savage MO (2014) Paediatric pituitary adenomas: a decade of change. Horm Res Paediatr 81:145–155
Herring N, Szmigielski C, Becher H, Karavitaki N, Wass JA (2009) Valvular heart disease and the use of cabergoline for the treatment of prolactinoma. Clin Endocrinol 70:104–108
Jane JA Jr, Laws ER Jr (2000) Surgical treatment of pituitary adenomas. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO (eds) Endotext. MDText.com, Inc., South Dartmouth
Kars M, Delgado V, Holman ER, Feelders RA, Smit JW, Romijn JA, Bax JJ, Pereira AM (2008) Aortic valve calcification and mild tricuspid regurgitation but no clinical heart disease after 8 years of dopamine agonist therapy for prolactinoma. J Clin Endocrinol Metab 93:3348–3356
Kharlip J, Salvatori R, Yenokyan G, Wand GS (2009) Recurrence of hyperprolactinemia after withdrawal of long-term cabergoline therapy. J Clin Endocrinol Metab 94:2428–2436
Klibanski A (2009) Dopamine agonist therapy in prolactinomas: when can treatment be discontinued? J Clin Endocrinol Metab 94:2247–2249
Kreutzer J, Buslei R, Wallaschofski H, Hofmann B, Nimsky C, Fahlbusch R, Buchfelder M (2008) Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol 158:11–18
Kunwar S, Wilson CB (1999) Pediatric pituitary adenomas. J Clin Endocrinol Metab 84:4385–4389
Lancellotti P, Livadariu E, Markov M, Daly AF, Burlacu MC, Betea D, Pierard L, Beckers A (2008) Cabergoline and the risk of valvular lesions in endocrine disease. Eur J Endocrinol 159:1–5
Maiter D, Delgrange E (2014) Therapy of endocrine disease: the challenges in managing giant prolactinomas. Eur J Endocrinol 170:R213–R227
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288
Muller HL (2013) Childhood craniopharyngioma. Pituitary 16:56–67
Muller HL (2014) Craniopharyngioma. Endocr Rev 35(3):513–543
Muller HL, Bueb K, Bartels U, Roth C, Harz K, Graf N, Korinthenberg R, Bettendorf M, Kuhl J, Gutjahr P, Sorensen N, Calaminus G (2001) Obesity after childhood craniopharyngioma—German multicenter study on pre-operative risk factors and quality of life. Klin Padiatr 213:244–249
Muller HL, Gebhardt U, Faldum A, Emser A, Etavard-Gorris N, Kolb R, Sorensen N (2005) Functional capacity and body mass index in patients with sellar masses—cross-sectional study on 403 patients diagnosed during childhood and adolescence. Childs Nerv Syst 21:539–545
Ono M, Miki N, Kawamata T, Makino R, Amano K, Seki T, Kubo O, Hori T, Takano K (2008) Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab 93:4721–4727
Sauder SE, Frager M, Case GD, Kelch RP, Marshall JC (1984) Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: responses to bromocriptine. J Clin Endocrinol Metab 59:941–948
Vallette S, Serri K, Rivera J, Santagata P, Delorme S, Garfield N, Kahtani N, Beauregard H, Aris-Jilwan N, Houde G, Serri O (2009) Long-term cabergoline therapy is not associated with valvular heart disease in patients with prolactinomas. Pituitary 12:153–157
Verhelst J, Abs R, Maiter D, van den Bruel A, Vandeweghe M, Velkeniers B, Mockel J, Lamberigts G, Petrossians P, Coremans P, Mahler C, Stevenaert A, Verlooy J, Raftopoulos C, Beckers A (1999) Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab 84:2518–2522
Wakil A, Rigby AS, Clark AL, Kallvikbacka-Bennett A, Atkin SL (2008) Low dose cabergoline for hyperprolactinaemia is not associated with clinically significant valvular heart disease. Eur J Endocrinol 159:R11–R14
Wang AT, Mullan RJ, Lane MA, Hazem A, Prasad C, Gathaiya NW, Fernandez-Balsells MM, Bagatto A, Coto-Yglesias F, Carey J, Elraiyah TA, Erwin PJ, Gandhi GY, Montori VM, Murad MH (2012) Treatment of hyperprolactinemia: a systematic review and meta-analysis. Syst Rev 1:33
Winters SJ, Troen P (1984) Altered pulsatile secretion of luteinizing hormone in hypogonadal men with hyperprolactinaemia. Clin Endocrinol 21:257–263
Wolff JE, Daumling E, Dirksen A, Dabrock A, Hartmann M, Jurgens H (1996) Munster Heidelberg abilities scale—a measuring instrument for global comparison of illness sequelae. Klin Padiatr 208:294–298
Acknowledgements
We are grateful for the help of Margarita Neff-Heinrich, Göttingen, Germany, in proofreading and editing the manuscript.
Funding
This study was funded by the German Childhood Cancer Foundation, Bonn, Germany (grant DKS 2014.13). The authors have no financial relationship with the organization that sponsored the research.
Author information
Authors and Affiliations
Contributions
Hoffmann A: Dr. Hoffmann has written and reviewed the manuscript and supervised data acquisition and analyses. She is the study assistant of the German Registry.
Adelmann S: Mrs. Adelmann analyzed retrospective data, and participated in writing and reviewing the manuscript.
Lohle K: Mrs. Lohle performed the statistical analyses, created the figures and tables, and participated in writing and reviewing the manuscript.
Claviez A: Dr. Claviez initiated the study, contributed patients, and participated in analyzing data and writing and reviewing the manuscript.
Müller HL: Dr. Müller is chairman of the German Registry. He supervised data collection, analyses, and participated in writing and review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Hermann L. Müller and coauthors declare that they have no conflict of interest.
This manuscript was composed in the absence of any commercial or financial relationships that could be perceived as a potential conflict of interest.
Ethical approval
All procedures performed in KRANIOPHARYNGEOM 2000—NCT00258453 and KRANIOPHARYNGEOM 2007—NCT01272622 involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the studies in KRANIOPHARYNGEOM 2000—NCT00258453 and KRANIOPHARYNGEOM 2007—NCT01272622.
Trial registration: KRANIOPHARYNGEOM 2000/2007 (NCT00258453; NCT01272622)
Additional information
Communicated by Peter de Winter
Rights and permissions
About this article
Cite this article
Hoffmann, A., Adelmann, S., Lohle, K. et al. Pediatric prolactinoma: initial presentation, treatment, and long-term prognosis. Eur J Pediatr 177, 125–132 (2018). https://doi.org/10.1007/s00431-017-3042-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-017-3042-5