Abstract
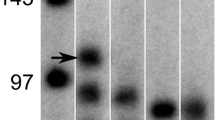
Congenital myopathies often have an unclear aetiology. Here, we studied a novel case of a severe congenital myopathy with a failure of myotube formation. Polymerase chain reaction-based analysis was performed to characterize the expression patterns of the Desmin, p21, p57, and muscle regulatory factors (MRFs) MyoD, Myf4, Myf5 and Myf6 in differentiating skeletal muscle cells (SkMCs), normal human fibroblasts and patient-derived fibroblasts during trans-differentiation. The temporal and spatial pattern of MRFs was further characterized by immunocyto- and immunohistochemical stainings. In differentiating SkMCs, each MRF showed a characteristic expression pattern. Normal trans-differentiating fibroblasts formed myotubes and expressed all of the MRFs, which were detected. Interestingly, the patient’s fibroblasts also showed some fusion events during trans-differentiation with a comparable expression profile for the MRFs, particularly, with increased expression of Myf4 and p21. Immunohistochemical analysis of normal and patient-derived skeletal musculature revealed that Myf4, which is downregulated during normal fetal development, was still present in patient-derived skeletal head muscle, which was also positive for Desmin and sarcomeric actin. The abnormal upregulation of Myf4 and p21 in the patient who suffered from a severe congenital myopathy suggests that the regulation of Myf4 and p21 gene expression during myogenesis might be of interest for further studies.








Similar content being viewed by others
Notes
Kindly provided by the Institute of Pathology, University of Freiburg.
References
Andrés V, Walsh K (1996) Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol 132:657–666
Arnold HH, Winter B (1998) Muscle differentiation: more complexity to the network of myogenic regulators. Curr Biol 8:539–544
Bailey P, Holowacz T, Lassar AB (2001) The origin of skeletal muscle stem cells in the embryo and the adult. Curr Opin Cell Biol 13:679–689
Bornemann A, Anderson LV (2000) Diagnostic protein expression in human muscle biopsies. Brain Pathol 10:193–214
Bornemann A, Goebel HH (2001) Congenital myopathies. Brain Pathol 11:206–217
Brand-Saberi B, Christ B (2000) Evolution and development of distinct cell lineages derived from somites. Curr Top Dev Biol 48:1–42
Braun T, Arnold HH (1995) Inactivation of Myf-6 and Myf-5 genes in mice leads to alterations in skeletal muscle development. EMBO J 14:1176–1186
Braun T, Rudnicki MA, Arnold HH, Jaenisch R (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71:369–382
Espinos E, Liu JH, Bader CR, Bernheim L (2001) Efficient non-viral DNA-mediated gene transfer to human primary myoblasts using electroporation. Neuromuscul Disord 11:341–349
Folpe AL (2002) MyoD1 and myogenin expression in human neoplasia: a review and update. Adv Anat Pathol 9:198–203
Fougerousse F, Durand M, Lopez S, Suel L, Demignon J, Thornton C, Ozaki H, Kawakami K, Barbet P, Beckmann J, Maire P (2002) Six and Eya expression during human somitogenesis and MyoD gene family activation. J Muscle Res Cell Motil 23:255–264
Hacker A, Guthrie S (1998) A distinct developmental programme for the cranial paraxial mesoderm in the chick embryo. Development 125:3461–3472
Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH (1993) Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364:501–506
Hawke TJ, Meeson AP, Jiang N, Graham S, Hutcheson K, DiMario JM, Garry DJ (2003) p21 is essential for normal myogenic progenitor cell function in regenerating skeletal muscle. Am J Physiol Cell Physiol 285:C1019—C1027
Kablar B, Krastel K, Tajbakhsh S, Rudnicki MA (2003) Myf5 and MyoD activation define independent myogenic compartments during embryonic development. Dev Biol 258:307–318
Kadi F, Johansson F, Johansson R, Sjöström M, Henriksson J (2004) Effects of one bout of endurance exercise on the expression of myogenin in human quadriceps muscle. Histochem Cell Biol 121:329–334
Kerst B, Mennerich D, Schuelke M, Stoltenburg-Didinger G, Moers A, Gossrau R, Landeghem FKH, Speer A, Braun T, Hübner C (2000) Heterozygous myogenic factor 6 mutation associated with myopathy and severe course of Becker muscular dystrophy. Neuromuscul Disord 10:572–577
Ketelsen UP (1999) Congenital myopathy with arrest of myogenesis before fusion of myoblasts to myotubes. Nervenheilkunde 7a:13–14
Ketelsen UP, Brand-Saberi B, Uhlenberg B, Wagner M, Laberke HG, Omran H (2005) Congenital myopathy with arrest of myogenesis prior to formation of myotubes. Neuropaediatrics 36:246–251
Mootoosamy RC, Dietrich S (2002) Distinct regulatory cascades for head and trunk myogenesis. Development 129:573–583
Muntoni F, Brown S, Sewry C, Patel K (2002) Muscle development genes: their relevance in neuromuscular disorders. Neuromuscul Disord 12:438–446
Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima YI (1993) Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364:532–535
Olivé M, Martinez-Matos JA, Ferrer I (1997) Expression of myogenic regulatory factors (MRFs) in human neuromuscular disorders. Neuropathol Appl Neurobiol 23:475–482
Parker MH, Seale P, Rudnicki MA (2003) Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nature 4:495–505
Perry RLS, Rudnicki MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5:750–767
Psilander N, Damsgaard R, Pilegaard H (2003) Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol 95:1038–1044
Rawls A, Morris JH, Rudnicki M, Braun T, Arnold HH, Klein WH, Olson EN (1995) Myogenin’s functions do not overlap with those of MyoD and Myf-5 during mouse embryogenesis. Dev Biol 172:37–50
Rawls A, Valdez MR, Zhang W, Richardson J, Klein WH, Olson EN (1998) Overlapping functions of the myogenin bHLH genes MRF4 and MyoD revealed in double mutant mice. Development 125:2349–2358
Reimann J, Brimah K, Schröder R, Wernig A, Beauchamp JR, Partridge TA (2004) Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tiss Res 315:233–242
Reynaud EG, Leibovitch MP, Tintignac LAJ, Pelpel K, Guillier M, Leibovitch SA (2000) Stabilization of MyoD by direct binding to p57Kip2. J Biol Chem 275:18767–18776
Ridgeway AG, Skerjanc IS (2001) Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J Biol Chem 276:19033–19039
Riggs JE, Bodensteiner JB, Schochet SS (2003) Congenital myopathies/dystrophies. Neurol Clin N Am 21:779–794
Rudnicki MA, Braun T, Hinuma S, Jaenisch R (1992) Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 71:383–390
Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75:1351–1359
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786
Shimokawa T, Kato M, Ezaki O, Hashimoto S (1998) Transcriptional regulation of muscle specific genes during myoblast differentiation. Biochem Biophys Res Commun 246:287–292
Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and myf-5 act upstream of MyoD. Cell 89:127–138
Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB (1988) MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science 242:405–411
Tseng BS, Cavin ST, Hoffmann EP, Iannaccone ST, Mancias P, Booth FW, Butler IJ (1999) Human bHLH transcription factor gene myogenin (MYOG): genomic sequence and negative mutation analysis in patients with severe congenital myopathies. Genomics 57:419–423
Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB (2003) Antagonists of Wnt and BMP signalling promote the formation of vertebrate and head muscle. Genes Dev 17:3087–3099
Valdez MR, Richardson JA, Klein WH, Olson EN (2000) Failure of Myf5 to support myogenic differentiation without myogenin, MyoD, and MRF4. Dev Biol 219:287–298
Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, Miller AD (1989) Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci USA 86:5434–5438
Weintraub H, Davis RL, Tapscott SJ, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar AB (1991) The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251:761–766
Zhang P, Behringer RR, Olson EN (1995) Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev 9:1388–1399
Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ (1997) Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith–Wiedemann syndrome. Nature 387:151–158
Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ (1999) p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev 13:213–224
Acknowledgments
Many thanks are dedicated to Prof. Anna Starzinski-Powitz and Dr Andreas Zeitvogel of the University of Frankfurt/Main for technical advice in handling the patient’s fibroblast as well as to the Institute of Pathology of the University of Freiburg providing normal fetal skeletal muscle tissue. We also express our thanks to Lidia Koschny for her excellent technical assistance. We acknowledge the support of the MYORES project (511978), funded by the EC’s Sixth Framework Programme.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to Professor Bodo Christ on the occasion of his 65th birthday.
Rights and permissions
About this article
Cite this article
Weise, C., Dai, F., Pröls, F. et al. Myogenin (Myf4) upregulation in trans-differentiating fibroblasts from a congenital myopathy with arrest of myogenesis and defects of myotube formation. Anat Embryol 211, 639–648 (2006). https://doi.org/10.1007/s00429-006-0117-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-006-0117-x