Abstract
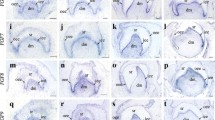
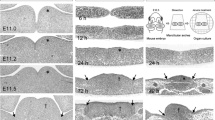
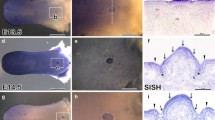
The Fgf/Fgfr (Fgf receptor) and Bmp signal pathways are critical for embryonic development and postnatal growth. In order to address their roles in tongue development, preliminary study of expression patterns of some important members in the two families, as well as of apoptosis and proliferation, were carried out in mouse developing tongue. Apoptosis in tongue is a very late event in embryogenesis, restricted to the upper layer of the epithelium whereas proliferation is very vigorous at the early stage of tongue development and remains active throughout embryogenesis. Bmp2, −4 and -5 were localized within the mesenchyme at the early embryonic stage of tongue development (E12 to E13), whereas Bmp3 and Bmp7 were mainly expressed in the epithelium. Most of these molecules were also seen in the tongue muscles at postnatal stages. Among Fgfr isoforms, Fgfr1c, −2b, and -2c were detected in embryogenesis with peak expression at E11 to E13. Fgfr1c and Fgfr2c were localized within the mesenchyme, while Fgfr2b was mainly expressed in the epithelium. High expression of Fgf7 and Fgf10 was also detected in the mesenchyme at the early embryonic stage of tongue development, corresponding to the Fgfr expression, suggesting that they are among the principal ligands functioning at the early embryonic expanding stage. Fgf2 was seen in the tongue muscles at the late embryonic and postnatal stages. These results suggest that Bmp and Fgf signalling regulates tongue development at multiple stages, possibly related to proliferation and differentiation.






Similar content being viewed by others
References
Aberg T, Wozney J, Thesleff I (1997) Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn 210(4):383–396
Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen Y (2005) The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev Biol 277(1):102–113
Amthor H, Christ B, Patel K (1999) A molecular mechanism enabling continuous embryonic muscle growth—a balance between proliferation and differentiation. Development 126(5):1041–1053
Brunetti A, Goldfine ID (1990) Role of myogenin in myoblast differentiation and its regulation by fibroblast growth factor. J Biol Chem 265(11):5960–5963
Couly GF, Coltey PM, Le Douarin NM (1992) The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 114(1):1–15
Dalrymple KR, Prigozy TI, Mayo M, Kedes L, Shuler CF (1999) Murine tongue muscle displays a distinct developmental profile of MRF and contractile gene expression. Int J Dev Biol 43(1):27–37
Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM (2001) Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet 27(1):84–88
De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C (2000) An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127(3):483–492
Duprez DM, Coltey M, Amthor H, Brickell PM, Tickle C (1996) Bone morphogenetic protein-2 (BMP-2) inhibits muscle development and promotes cartilage formation in chick limb bud cultures. Dev Biol 174(2):448–452
Eswarakumar VP, Monsonego-Ornan E, Pines M, Antonopoulou I, Morriss-Kay GM, Lonai P (2002) The IIIc alternative of Fgfr2 is a positive regulator of bone formation. Development 129(16):3783–3793
Flanagan-Steet H, Hannon K, McAvoy MJ, Hullinger R, Olwin BB (2000) Loss of FGF receptor 1 signaling reduces skeletal muscle mass and disrupts myofiber organization in the developing limb. Dev Biol 218(1):21–37
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119(3):493–501
Hannon K, Kudla AJ, McAvoy MJ, Clase KL, Olwin BB (1996) Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J Cell Biol 132(6):1151–1159
Huang R, Zhi Q, Izpisua-Belmonte JC, Christ B, Patel K (1999) Origin and development of the avian tongue muscles. Anat Embryol (Berl) 200(2):137–152
Huang R, Lang ER, Otto WR, Christ B, Patel K (2001) Molecular and cellular analysis of embryonic avian tongue development. Anat Embryol (Berl) 204(3):179–187
Jung HS, Oropeza V, Thesleff I (1999) Shh, Bmp-2, Bmp-4 and Fgf-8 are associated with initiation and patterning of mouse tongue papillae. Mech Dev 81(1–2):179–182
Kettunen P, Karavanova I, Thesleff I (1998) Responsiveness of developing dental tissues to fibroblast growth factors: expression of splicing alternatives of FGFR1, −2, −3, and of FGFR4; and stimulation of cell proliferation by FGF-2, −4, −8, and −9. Dev Genet 22(4):374–385
Kettunen P, Laurikkala J, Itaranta P, Vainio S, Itoh N, Thesleff I (2000) Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev Dyn 219(3):322–332
Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S (2002) Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 129(1):53–60
Musgrave DS, Pruchnic R, Wright V, Bosch P, Ghivizzani SC, Robbins PD, Huard J (2001) The effect of bone morphogenetic protein-2 expression on the early fate of skeletal muscle-derived cells. Bone 28(5):499–506
Nagata J, Yamane A (2004) Progress of cell proliferation in striated muscle tissues during development of the mouse tongue. J Dent Res 83(12):926–929
Noden DM (1991) Cell movements and control of patterned tissue assembly during craniofacial development. J Craniofac Genet Dev Biol 11(4):192–213
Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N (2000) FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277(3):643–649
Ornitz DM, Itoh N (2001) Fibroblast growth factors. Genome Biol 2(3), REVIEWS3005
Patel SG, Funk PE, DiMario JX (1999) Regulation of avian fibroblast growth factor receptor 1 (FGFR-1) gene expression during skeletal muscle differentiation. Gene 237(1):265–276
Scata KA, Bernard DW, Fox J, Swain JL (1999) FGF receptor availability regulates skeletal myogenesis. Exp Cell Res 250(1):10–21
Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21(1):138–141
Shuler CF, Dalrymple KR (2001) Molecular regulation of tongue and craniofacial muscle differentiation. Crit Rev Oral Biol Med 12(1):3–17
Singh DJ, Bartlett SP (2005) Congenital mandibular hypoplasia: analysis and classification. J Craniofac Surg 16(2):291–300
Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB (2003) Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17(24):3087–3099
Vainio S, Karavanova I, Jowett A, Thesleff I (1993) Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75(1):45–58
Vogel JE, Mulliken JB, Kaban LB (1986) Macroglossia: a review of the condition and a new classification. Plast Reconstr Surg 78(6):715–723
Wilkinson DG, Green J (1990) In situ hybridization and the three-dimensional reconstruction of serial sections. In: Copp AJ, Cockroft DL (eds) Postimplantation mammalian embryos: a practical approach. IRL Press, Oxford, pp 155–171
Yamane A, Mayo M, Shuler C, Crowe D, Ohnuki Y, Dalrymple K, Saeki Y (2000) Expression of myogenic regulatory factors during the development of mouse tongue striated muscle. Arch Oral Biol 45(1):71–78
Zhao P, Hoffman EP (2004) Embryonic myogenesis pathways in muscle regeneration. Dev Dyn 229(2):380–392
Acknowledgements
This work was carried out in the department of Biomedicine and supported by Faculty of Dentistry, University of Bergen. I would like to express my thanks to Kjellfrid Haukanes and Anne Nyhaug for their skilful technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nie, X. Apoptosis, proliferation and gene expression patterns in mouse developing tongue. Anat Embryol 210, 125–132 (2005). https://doi.org/10.1007/s00429-005-0009-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-005-0009-5