Abstract
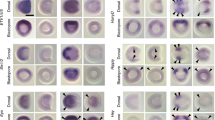
We have isolated an amphioxus T-box gene that is orthologous to the two vertebrate genes, Tbx1 and Tbx10, and examined its expression pattern during embryonic and early larval development. AmphiTbx1/10 is first expressed in branchial arch endoderm and mesoderm of developing neurulae, and in a bilateral, segmented pattern in the ventral half of newly formed somites. Branchial expression is restricted to the first three branchial arches, and disappears completely by 4 days post fertilization. Ventral somitic expression is restricted to the first 10–12 somites, and is not observed in early larvae except in the most ventral mesoderm of the first three branchial arches. No expression can be detected by 4 days post fertilization. Integrating functional, phylogenetic and expression data from amphioxus and a variety of vertebrate model organisms, we have reconstructed the early evolutionary history of the Tbx1/10 subfamily of genes within the chordate lineage. We conclude that Tbx1/10-mediated branchial arch endoderm and mesoderm patterning functions predated the origin of neural crest, and that ventral somite specification functions predated the origin of vertebrate sclerotome, but that Tbx1 was later co-opted during the evolution of developmental programs regulating branchial neural crest and sclerotome migration.



Similar content being viewed by others
References
Abouheif E, Akam M, Dickinson WJ, Holland PWH, Meyer A, Patel NH, Raff RA, Roth VL, Wray GA (1997) Homology and developmental genes. Trends Genet 13:432–433
Ataliotis P, Latinkic B, Mohun TJ, Scambler PJ (2001) Analysis of Tbx1 function in Xenopus laevis. Dev Biol 235:245
Bollag R, Siegfried Z, Cebra-Thomas JA, Garvey N, Davison EM, Silver LM (1994) An ancient family of embryonically expressed mouse genes sharing a conserved protein motif with the T locus. Nat Genet 7:383–389
Bush JO, Maltby KM, Cho E-C, Jiang R (2003) The T-box gene Tbx10 exhibits a uniquely restricted expression pattern during mouse embryogenesis. Gene Expr Patterns 3:533–538
Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE (1996) Expression of the T-box family genes, Tbx1-it Tbx5, during early mouse development. Dev Dyn 206:379–390
Chieffo C, Garvey N, Gong W, Roe B, Zhang G, Silver L, Emanuel BS, Budarf ML (1997) Isolation and characterization of a gene from the DiGeorge chromosomal region homologous to the mouse Tbx1 gene. Genomics 43:267–277
Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM et al (2002) The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science 298:2157–2167
Furlong RF, Holland PWH (2002) Were vertebrates octaploid? Philos Trans R Soc Lond B Biol Sci 357:531–544
Garg V, Yamagashi C, Hu T, Kathiriya IS, Yamagashi H, Srivastava D (2001) Tbx1, a DiGeorge syndrome candidate gene, is regulated by Sonic Hedgehog during pharyngeal arch development. Dev Biol 235:62–73
Gibson-Brown JJ (2002) T-box time in England. Dev Cell 3:625–630
Hall BK (1994) Homology: the hierachical basis of comparative biology. Academic, San Diego
Hall BK (2003) Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development. Biol Rev Camb Philos Soc 78:409–433
Holland ND, Holland LZ (1993) Embryos and larvae of invertebrate deuterostomes. In Stern CD, Holland PWH (eds) Essential developmental biology: a practical approach. IRL Press, Oxford, pp 21–32
Holland LZ, Holland PWH, Holland ND (1996) Revealing homologies between body parts of distantly related animals by in situ hybridization to developmental genes: amphioxus versus vertebrates. In: Ferraris JD, Palumbi SR (eds) Molecular zoology: advances, strategies, and protocols. Wiley-Liss, New York, pp 267–282, 473–483
Horton AC, Mahadevan NR, Ruvinsky I, Gibson-Brown JJ (2003) Phylogenetic analyses alone are insufficient to determine whether whole-genome duplication(s) occurred during early vertebrate evolution. J Exp Zool Part B Mol Dev Evol 299:41–53
Jerome LA, Papioannou VE (2001) DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet 27:286–291
Langeland JA, Tomsa JM, Jackman WR Jr, Kimmel CB (1998) An amphioxus snail gene: expression in paraxial mesoderm and neural plate suggests a conserved role in patterning the chordate embryo. Dev Genes Evol 208:569–577
Law DJ, Garvey N, Agulnik SI, Perlroth V, Hahn OM, Rhinehart RE, Gehbuhr TC, Silver LM (1998) TBX10, a member of the Tbx1-subfamily of conserved developmental genes, is located at human chromosome 11q13 and proximal chromosome 19. Mamm Genome 9:397–399
Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A (2001) Tbx1 haploinsufficiency in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 410:97–101
Merscher S, Funke B, Epstein JA, Heyer J, Peuch A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore B St, Lopez M, Pandita RK, Lia M, Carrion M, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R (2001) TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 104:619–629
Ohno S (1970) Evolution by gene duplication. Springer, Berlin Heidelberg New York
Papaioannou VE (2001) T-box genes in development: from hydra to humans. Int Rev Cytol 207:1–70
Piotrowski T, Nüesslein-Volhard C (2000) The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev Biol 225:339–356
Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, Foott H, Dawid IB, Ho RK (2003) The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development 130:5043–5052
Porsch M, Hofmeyer K, Bausenwein BS, Grimm S, Weber BHF, Miassod R, Pflugfelder GO (1998) Isolation of a Drosophila T-it box gene closely related to human TBX1. Gene 212:237–248
Raft S, Nowotschin S, Liao J, Morrow BE (2004) Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development 131:1801–1812
Ruvinsky I, Silver LM, Gibson-Brown JJ (2000) Phylogenetic analysis of T-box genes demonstrates the importance of amphioxus for understanding evolution of the vertebrate genome. Genetics 156:1249–1257
Sauka-Spengler T, Le Mentec C, Lepage M, Mazan S (2002) Embryonic expression of Tbx1 a DiGeorge syndrome candidate gene, in the lamprey Lampetra fluviatilis. Gene Expr Patterns 2:99–103
Schinke A, Izumo S (2001) Deconstructing DiGeorge syndrome. Nat Genet 27:238–240
Simon H-G, Kittappa R, Khan PA, Tsilfidis C, Liversage RA, Oppenheimer S (1997) A novel family of T-box genes in urodele amphibian limb development and regeneration: candidate genes involved in vertebrate forelimb/hindlimb patterning. Development 124:1355–1366
Stone JR, Hall BK (2004) Latent homologues for the neural crest as an evolutionary novelty. Evol Dev 6:123–129
Strimmer K, von Haeseler A (1997) Likelihood-mapping: a simple method to visualize phylogenetic content of a sequence alignment. Proc Natl Acad Sci USA 94:6815–6819
Swofford DL (2001) PAUP* beta 5: phylogenetic analysis using parsimony (and other methods). Sinauer, Sunderland, Mass.
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vitelli F, Morishima M, Taddei I, Lindsay EA, Baldini A (2002a) Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet 11:915–922
Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A (2002b) A genetic link between Tbx1 and fibroblast growth factor signaling. Development 129:4605–4611
Yamagishi H, Maeda J, Hu T, McAnelly J, Conway SJ, Kume T, Meyers EN, Yamagishi C, Srivastava D (2003) Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev 17:149–281
Acknowledgements
We wish to thank Nick and Linda Holland for instruction in the collection of amphioxus embryos and whole-mount in situ hybridization, Jim Langeland for the amphioxus cDNA library, John Lawrence and Skip Pierce for providing laboratory space at the University of South Florida, Amy Horton and Ilya Ruvinsky for advice on phylogenetic analyses, Mike Veith for instruction in cutting plastic sections, and Paris Ataliotis for providing access to his unpublished Xenopus Tbx1 sequence. Finally, we are most grateful to Lee Silver, in whose laboratory at Princeton these studies were initiated, for his generous support. This work was supported by NIH grant HD-20275 to Lee M. Silver, a Beckman Scholar award to N.M., a Development Traveling Fellowship from The Company of Biologists, and departmental support from Washington University to J.J.G.-B.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M. Akam
Rights and permissions
About this article
Cite this article
Mahadevan, N.R., Horton, A.C. & Gibson-Brown, J.J. Developmental expression of the amphioxus Tbx1/10 gene illuminates the evolution of vertebrate branchial arches and sclerotome. Dev Genes Evol 214, 559–566 (2004). https://doi.org/10.1007/s00427-004-0433-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-004-0433-1