Abstract
Main conclusion
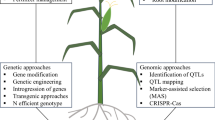
This review presents genetic and molecular basis of crop height using a rice crop model. Height is controlled by multiple genes with potential to be manipulated through breeding strategies to improve productivity.
Height is an important factor affecting crop architecture, apical dominance, biomass, resistance to lodging, tolerance to crowding and mechanical harvesting. The impressive increase in wheat and rice yield during the ‘green revolution’ benefited from a combination of breeding for high-yielding dwarf varieties together with advances in agricultural mechanization, irrigation and agrochemical/fertilizer use. To maximize yield under irrigation and high fertilizer use, semi-dwarfing is optimal, whereas extreme dwarfing leads to decreased yield. Rice plant height is controlled by genes that lie in a complex regulatory network, mainly involved in the biosynthesis or signal transduction of phytohormones such as gibberellins, brassinosteroids and strigolactones. Additional dwarfing genes have been discovered that are involved in other pathways, some of which are uncharacterized. This review discusses our current understanding of the regulation of plant height using rice as a well-characterized model and highlights some of the most promising research that could lead to the development of new, high-yielding varieties. This knowledge underpins future work towards the genetic improvement of plant height in rice and other crops.








Similar content being viewed by others
References
Abe S, Sado A, Tanaka K, Kisugi T, Asami K, Ota S, Kim HI, Yoneyama K, Xie X, Ohnishi T, Seto Y, Yamaguchi S, Akiyama K, Yoneyama K, Nomura T (2014) Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc Nati Acad Sci USA 11:18084–18089. doi:10.1073/pnas.1410801111
Achard P, Genschik P (2009) Releasing the brakes of plant growth: how GAs shutdown DELLA proteins. J Exp Bot 60:1085–1092. doi:10.1093/jxb/ern301
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827. doi:10.1038/nature03608
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science (New York, N Y) 335:1348–1351. doi:10.1126/science.1218094
Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51:1019–1029. doi:10.1111/j.1365-313X.2007.03210.x
Asano K, Hirano K, Ueguchitanaka M, Angelesshim RB, Komura T, Satoh H, Kitano H, Matsuoka M, Ashikari M (2009) Isolation and characterization of dominant dwarf mutants, Slr1-d, in rice. Mol Genet Genom 281:223–231
Asano K, Miyao A, Hirochika H, Kitano H, Matsuoka M, Ashikari M (2010) SSD1, which encodes a plant-specific novel protein, controls plant elongation by regulating cell division in rice. Proc Jpn Acad B Phys 86:265–273
Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein. Proc Nati Acad Sci USA 96:10284–10289
Ashikari M, Sasaki A, Ueguchi-Tanaka M, Itoh H, Nishimura A, Datta S, Ishiyama K, Saito T, Kobayashi M, Khush GS (2002) Loss-of-function of a rice gibberellin biosynthetic gene, GA20 oxidase (GA20ox-2), led to the rice’ green revolution’. Breed Sci 52:143–150
Ayano M, Kani T, Kojima M, Sakakibara H, Kitaoka T, Kuroha T, Angelesshim RB, Kitano H, Nagai K, Ashikari M (2015) Gibberellin biosynthesis and signal transduction is essential for internode elongation in deepwater rice. Plant Cell Environ 37:2313–2324
Bai MY, Zhang LY, Gampala SS, Zhu SW, Song WY, Chong K, Wang ZY (2007) Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Nati Acad Sci USA 104:13839–13844. doi:10.1073/pnas.0706386104
Begonia GB, Begonia MT (2007) Plant photosynthetic production as controlled by leaf growth, phenology, and behavior. Photosynthetica 45:321–333
Beharav A, Cahaner A, Pinthus M (1998) Genetic correlations between culm length, grain yield and seedling elongation within tall (rht1) and semi-dwarf (Rht1) spring wheat (Trificum aestivum L.). Eur J Agron 9:35–40
Beveridge CA, Kyozuka J (2010) New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 13:34–39. doi:10.1016/j.pbi.2009.10.003
Bolle C (2004) The role of GRAS proteins in plant signal transduction and development. Planta 218:683–692. doi:10.1007/s00425-004-1203-z
Bonnett D, Ellis M, Rebetzke G, Condon A, Spielmeyer W, Richards R (2001) Dwarfing genes in Australian wheat—present and future. In: Proceedings of the 10th Australian wheat breeders assembly, Mildura, Australia, pp 154–157
Botwright TL, Rebetzke GJ, Condon AG, Richards RA (2005) Influence of the gibberellin-sensitive Rht8 dwarfing gene on leaf epidermal cell dimensions and early vigour in wheat (Triticum aestivum L.). Ann Bot 95:631–639. doi:10.1093/aob/mci069
Butany W, Bhattacharyya R, Daiya L (1959) Inheritance of dwarf character in rice and its interrelationship with the occurrence of anthocyanin pigment in various plant parts. Indian J Genet Plant Breed 19:64–72
Butler JD, Byrne PF, Mohammadi V, Chapman PL, Haley SD (2005) Agronomic performance of Rht alleles in a spring wheat population across a range of moisture levels. Crop Sci 45:939–947
Chang T, Zuro C, Marciano R, Loresto G (1984) Semidwarfing genes in rice germplasm collection. Rice Genet Newsl 1:93–94
Chapman SC, Mathews KL, Trethowan RM, Singh RP (2007) Relationships between height and yield in near-isogenic spring wheats that contrast for major reduced height genes. Euphytica 157:391–397
Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, Feldmann KA (2001) Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J 26:573–582
Clouse SD (2002) Brassinosteroid signal transduction: clarifying the pathway from ligand perception to gene expression. Mol Cell 10:973–982
Dai M, Zhao Y, Ma Q, Hu Y, Hedden P, Zhang Q, Zhou DX (2007) The rice YABBY1 gene is involved in the feedback regulation of gibberellin metabolism. Plant Physiol 144:121–133. doi:10.1104/pp.107.096586
Davière JM, Achard P (2013) Gibberellin signaling in plants. Development 140:1147–1151
Di Rubbo RS, Irani NG, Russinova E (2011) PP2A phosphatases: the “on–off” regulatory switches of brassinosteroid signaling. Sci Signal 4:pe25
Diaz J (2016) Characterization of the role of LOB-DOMAIN genes and brassinosteroids in rice architecture. UC Riverside Electronic Theses and Dissertations
Divi UK, Krishna P (2009) Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol 26:131–136. doi:10.1016/j.nbt.2009.07.006
Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12:211–221. doi:10.1038/nrm3088
Emerson RA, Beadle GW, Fraser AC (1935) A summary of linkage studies in maize. Cornell Univ Agric Exp Stn Mem 180:1–83
Fischer RA, Quail KJ (1990) The effect of major dwarfing genes on yield potential in spring wheats. Euphytica 46:51–56. doi:10.1007/bf00057618
Flintham JE, Borner A, Worland AJ, Gale MD (1997) Optimizing wheat grain yield: effects of Rht (gibberellin-insensitive) dwarfing genes. J Agric Sci 128:11–25
Foisset N, Delourme R, Barret P, Renard M (1995) Molecular tagging of the dwarf BREIZH (Bzh) gene in Brassica napus. Theor Appl Genet 91:756–761. doi:10.1007/bf00220955
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5:344–350
Foster AE, Thompson AP (1987) Effects of a semidwarf gene from Jotun on agronomic and quality traits of barley. In: Yasuda S, Kanishi T (eds) Proceedings of the 5th international barley genetics symposium, 1986. Sanyo, Okayama, pp 979–982
Fujioka S, Sakurai A (1997) Brassinosteroids. Nat Prod Rep 14:1–10
Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54:137–164
Fukazawa J, Teramura H, Murakoshi S, Nasuno K, Nishida N, Ito T, Yoshida M, Kamiya Y, Yamaguchi S, Takahashi Y (2014) DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 26:2920–2938
Fukazawa J, Ito T, Kamiya Y, Yamaguchi S, Takahashi Y (2015) Binding of GID1 to DELLAs promotes dissociation of GAF1 from DELLA in GA dependent manner. Plant Sig Beha 10:e1052923-3
Futsuhara Y, Kikuchi F (1997) Inheritance of morphological characters. 2. Inheritance of dwarfism. Sci Rice Plant 3:300–308
Gao Z, Qian Q, Liu X, Yan M, Feng Q, Dong G, Liu J, Han B (2009) Dwarf 88, a novel putative esterase gene affecting architecture of rice plant. Plant Mol Biol 71:265–276. doi:10.1007/s11103-009-9522-x
Gao X, Chen Z, Zhang J, Li X, Chen G, Li X, Wu C (2012) OsLIS-L1 encoding a lissencephaly type-1-like protein with WD40 repeats is required for plant height and male gametophyte formation in rice. Planta 235:713–727. doi:10.1007/s00425-011-1532-7
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Gomi K, Sasaki A, Itoh H, Ueguchi-Tanaka M, Ashikari M, Kitano H, Matsuoka M (2004) GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant J 37:626–634
Grove MD, Spencer GF, Rohwedder WK, Mandava N, Worley JF, Warthen JD, Steffens GL, Flippen-Anderson JL, Cook JC (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217
Gui J, Zheng S, Liu C, Shen J, Li J, Li L (2016) OsREM4.1 interacts with OsSERK1 to coordinate the interlinking between abscisic acid and brassinosteroid signaling in rice. Dev Cell 38:201–213
Guo S, Xu Y, Liu H, Mao Z, Zhang C, Ma Y, Zhang Q, Meng Z, Chong K (2013) The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat Commun 4:1566. doi:10.1038/ncomms2542
Harberd NP (2003) Botany. Relieving DELLA restraint. Science (New York, N Y) 299:1853–1854. doi:10.1126/science.1083217
Harberd NP, Belfield E, Yasumura Y (2009) The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21:1328–1339. doi:10.1105/tpc.109.066969
Hargrove TR, Cabanilla VL (1979) The impact of semidwarf varieties on Asian rice-breeding programs. BioScience 29:731–735
Harrison PJ, Newgas SA, Descombes F, Shepherd SA, Thompson AJ, Bugg TD (2015) Biochemical characterization and selective inhibition of β-carotene cis–trans isomerase D27 and carotenoid cleavage dioxygenase CCD8 on the strigolactone biosynthetic pathway. FEBS J 282:3986–4000
He Z, Shen Z (1991) Inheritance of panicle exsertion and improvement of male sterile line in rice. Chin J Rice Sci 5:1–6
Hedden Peter (2003) The genes of the green revolution. Trends Genet 19:5–9
Hedden P (2010) Green revolution genes. Rothamsted Research, Harpenden, Hertfordshire, UK Essay 20.3
Hedden P, Kamiya Y (1997) GIBBERELLIN BIOSYNTHESIS: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol 48:431–460. doi:10.1146/annurev.arplant.48.1.431
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530
Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444:11–25
Hirano K, Asano K, Tsuji H, Kawamura M, Mori H, Kitano H, Ueguchi-Tanaka M, Matsuoka M (2010) Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice. Plant Cell 22:2680–2696. doi:10.1105/tpc.110.075549
Hirano K, Kouketu E, Katoh H, Aya K, Ueguchi-Tanaka M, Matsuoka M (2012) The suppressive function of the rice DELLA protein SLR1 is dependent on its transcriptional activation activity. Plant J 71:443–453. doi:10.1111/j.1365-313X.2012.05000.x
Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agetsuma M, Yoshida S, Watanabe Y, Uozu S, Kitano H, Ashikari M, Matsuoka M (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32:495–508
Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M (2003) A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15:2900–2910. doi:10.1105/tpc.014712
Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) The Rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 17:2243–2254. doi:10.1105/tpc.105.030973
Hu X, Qian Q, Xu T, Zhang Y, Dong G, Gao T, Xie Q, Xue Y (2013) The U-box E3 ubiquitin ligase TUD1 functions with a heterotrimeric G alpha subunit to regulate brassinosteroid-mediated growth in rice. PLoS Genet 9:e1003391. doi:10.1371/journal.pgen.1003391
Hu Z, Yamauchi T, Yang J, Jikumaru Y, TsuchidaMayama T, Ichikawa H, Takamure I, Nagamura Y, Tsutsumi N, Yamaguchi S (2014) Strigolactone and cytokinin act antagonistically in regulating rice mesocotyl elongation in darkness. Plant Cell Physiol 55:30–41
Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Xia G, Chu C, Li J, Fu X (2009) Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet 41:494–497
Huang J, Tang D, Shen Y, Qin B, Hong L, You A, Li M, Wang X, Yu H, Gu M, Cheng Z (2010) Activation of gibberellin 2-oxidase 6 decreases active gibberellin levels and creates a dominant semi-dwarf phenotype in rice (Oryza sativa L.). J Genet Genom 37:23–36. doi:10.1016/s1673-8527(09)60022-9
Ikeda A, Ueguchi-Tanaka M, Sonoda Y, Kitano H, Koshioka M, Futsuhara Y, Matsuoka M, Yamaguchi J (2001) slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13:999–1010
Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46:79–86. doi:10.1093/pcp/pci022
Itoh H, Ueguchi-Tanaka M, Sentoku N, Kitano H, Matsuoka M, Kobayashi M (2001) Cloning and functional analysis of two gibberellin 3 beta -hydroxylase genes that are differently expressed during the growth of rice. Proc Nati Acad Sci USA 98:8909–8914. doi:10.1073/pnas.141239398
Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, Kitano H, Ashikari M, Ichihara S, Matsuoka M (2004) A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol 54:533–547. doi:10.1023/b:plan.0000038261.21060.47
Iwata N, Satoh H, Omura T (1977) Linkage studies in rice. Linkage groups for 6 genes newly described. Jpn J Breed 27:250–251
Iwata N, Takamure I, Wu H, Siddinq E, Rutger J (1995) List of genes for various traits (with chromosome and main literature). Rice Genet Newsl 12:61–93
Jiang Y, Bao L, Jeong SY, Kim SK, Xu C, Li X, Zhang Q (2012) XIAO is involved in the control of organ size by contributing to the regulation of signaling and homeostasis of brassinosteroids and cell cycling in rice. Plant J 70:398–408. doi:10.1111/j.1365-313X.2011.04877.x
Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, Yi W, Zhao L, Ma H, He Y, Wu Z, Melcher K, Qian Q, Xu HE, Wang Y, Li J (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504:401–405. doi:10.1038/nature12870
Kameoka H, Kyozuka J (2015) Downregulation of rice DWARF 14 LIKE suppress mesocotyl elongation via a strigolactone independent pathway in the dark. J Genet Genom 42:119–124
Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Nat Acad Sci 105:7100–7105
Khush GS (1999) Green revolution: preparing for the 21st century. Genome 42:646–655
Kim SR, Yang JI, Moon S, Ryu CH, An K, Kim KM, Yim J, An G (2009) Rice OGR1 encodes a pentatricopeptide repeat-DYW protein and is essential for RNA editing in mitochondria. Plant J 59:738–749. doi:10.1111/j.1365-313X.2009.03909.x
Kinoshita T (1995) Report of committee on gene symbolization, nomenclature and linkage groups. Rice Genet Newsl 12:9–153
Koh S, Lee SC, Kim MK, Koh JH, Lee S, An G, Choe S, Kim SR (2007) T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol Biol 65:453–466. doi:10.1007/s11103-007-9213-4
Kovi MR, Zhang Y, Yu S, Yang G, Yan W, Xing Y (2011) Candidacy of a chitin-inducible gibberellin-responsive gene for a major locus affecting plant height in rice that is closely linked to green revolution gene sd1. Theor Appl Genet 123:705–714. doi:10.1007/s00122-011-1620-x
Kwon M, Fujioka S, Jeon JH, Kim HB, Takatsuto S, Yoshida S, An CS, Choe S (2005) A double mutant for the CYP85A1 and CYP85A2 genes of Arabidopsis exhibits a brassinosteroid dwarf phenotype. J Plant Biol 48:237–244
Lawit SJ, Wych HM, Xu D, Kundu S, Tomes DT (2010) Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development. Plant Cell Physiol 51:1854–1868
Lee DJ, Zeevaart JA (2005) Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris. Plant Physiol 138:243–254. doi:10.1104/pp.104.056499
Leyser O (2009) The control of shoot branching: an example of plant information processing. Plant Cell Environ 32:694–703. doi:10.1111/j.1365-3040.2009.01930.x
Li JM, Yuan LP (2000) Hybrid rice: genetics, breeding, and seed production. Plant Breed Rev 17:15–158
Li F, Asami T, Wu X, Tsang EW, Cutler AJ (2007) A putative hydroxysteroid dehydrogenase involved in regulating plant growth and development. Plant Physiol 145:87–97. doi:10.1104/pp.107.100560
Li M, Xiong G, Li R, Cui J, Tang D, Zhang B, Pauly M, Cheng Z, Zhou Y (2009) Rice cellulose synthase-like D4 is essential for normal cell-wall biosynthesis and plant growth. Plant J 60:1055–1069. doi:10.1111/j.1365-313X.2009.04022.x
Li H, Jiang L, Youn JH, Sun W, Cheng Z, Jin T, Ma X, Guo X, Wang J, Zhang X, Wu F, Wu C, Kim SK, Wan J (2013) A comprehensive genetic study reveals a crucial role of CYP90D2/D2 in regulating plant architecture in rice (Oryza sativa). New Phytol 200:1076–1088. doi:10.1111/nph.12427
Li H, Chen G, Yan W (2015) Molecular Characterization of barley 3H semi-dwarf genes. PLoS One 10:e0120558
Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, Wang Y (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21:1512–1525. doi:10.1105/tpc.109.065987
Liu B, Wu Y, Fu X, Qian Q (2008) Characterizations and molecular mapping of a novel dominant semi-dwarf gene Sdd (t) in rice (Oryza sativa). Plant Breed 127:125–130
Liu W, Wu C, Fu Y, Hu G, Si H, Zhu L, Luan W, He Z, Sun Z (2009) Identification and characterization of HTD2: a novel gene negatively regulating tiller bud outgrowth in rice. Planta 230:649–658. doi:10.1007/s00425-009-0975-6
Liu Y, Xu Y, Xiao J, Ma Q, Li D, Xue Z, Chong K (2011) OsDOG, a gibberellin-induced A20/AN1 zinc-finger protein, negatively regulates gibberellin-mediated cell elongation in rice. J Plant Physiol 168:1098–1105. doi:10.1016/j.jplph.2010.12.013
Liu HL, Yin ZJ, Xiao L, Xu YN, le Qu Q (2012) Identification and evaluation of omega-3 fatty acid desaturase genes for hyperfortifying alpha-linolenic acid in transgenic rice seed. J Exp Bot 63:3279–3287. doi:10.1093/jxb/ers051
Lo SF, Yang SY, Chen KT, Hsing YI, Zeevaart JA, Chen LJ, Yu SM (2008) A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 20:2603–2618. doi:10.1105/tpc.108.060913
Luan W, Liu Y, Zhang F, Song Y, Wang Z, Peng Y, Sun Z (2011) OsCD1 encodes a putative member of the cellulose synthase-like D sub-family and is essential for rice plant architecture and growth. Plant Biotechnol J 9:513–524. doi:10.1111/j.1467-7652.2010.00570.x
Luo A, Qian Q, Yin H, Liu X, Yin C, Lan Y, Tang J, Tang Z, Cao S, Wang X, Xia K, Fu X, Luo D, Chu C (2006) EUI1, encoding a putative cytochrome P450 monooxygenase, regulates internode elongation by modulating gibberellin responses in rice. Plant Cell Physiol 47:181–191. doi:10.1093/pcp/pci233
Marcia MP, Zhou XR, Zhu QH, Dennis ES, Upadhyaya NM (2005) Isolation and characterization of a Ds-tagged rice (Oryza sativa L.) GA-responsive dwarf mutant defective in an early step of the gibberellin biosynthesis pathway. Plant Cell Rep 23:819–833. doi:10.1007/s00299-004-0896-6
Mcintosh R, Hart G, Gale M (1988) Catalogue of gene symbols for wheat : 1988 supplement. Cereal Res Commun 121–135
Miralles D, Slafer G (1995) Yield, biomass and yield components in dwarf, semi-dwarf and tall isogenic lines of spring wheat under recommended and late sowing dates. Plant Breed 114:392–396
Mitsunaga S, Tashiro T, Yamaguchi J (1994) Identification and characterization of gibberellin-insensitive mutants selected from among dwarf mutants of rice. TAG Theor Appl Genet 87:705–712. doi:10.1007/bf00222896
Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M, Sato M, Nasu S, Minobe Y (2002) Positional cloning of rice semi-dwarfing gene, sd-1: rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res 9:11–17
Mori M, Nomura T, Ooka H, Ishizaka M, Yokota T, Sugimoto K, Okabe K, Kajiwara H, Satoh K, Yamamoto K, Hirochika H, Kikuchi S (2002) Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol 130:1152–1161. doi:10.1104/pp.007179
Muangprom A, Osborn TC (2004) Characterization of a dwarf gene in Brassica rapa, including the identification of a candidate gene. Theor Appl Genet 108:1378–1384. doi:10.1007/s00122-003-1551-2
Murai M, Shinbashi N, Kusutani A, Hirose S, Takamure I, Kinoshita T (1990) Identification of dwarf genes in rice lines, Yukara dwarf and Fukei 71, and its response to environmental factors. Jpn J Breed 40:33–45
Nakagawa H, Tanaka A, Tanabata T, Ohtake M, Fujioka S, Nakamura H, Ichikawa H, Mori M (2012) Short grain1 decreases organ elongation and brassinosteroid response in rice. Plant Physiol 158:1208–1219. doi:10.1104/pp.111.187567
Neuffer MG, Coe EH, Wessler SR (1997) Mutants of maize. Cold Spring Harbor Laboratory Press, New York, pp 1–255
Ogawa S, Toyomasu T, Yamane H, Murofushi N, Ikeda R, Morimoto Y, Nishimura Y, Omori T (1996) A step in the biosynthesis of gibberellins that is controlled by the mutation in the semi-dwarf rice cultivar Tan-Ginbozu. Plant Cell Physiol 37:363–368
Ogawa M, Kusano T, Katsumi M, Sano H (2000) Rice gibberellin-insensitive gene homolog, OsGAI, encodes a nuclear-localized protein capable of gene activation at transcriptional level. Gene 245:21–29
Ohnishi T, Szatmari AM, Watanabe B, Fujita S, Bancos S, Koncz C, Lafos M, Shibata K, Yokota T, Sakata K, Szekeres M, Mizutani M (2006) C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 18:3275–3288. doi:10.1105/tpc.106.045443
Ohnishi T, Godza B, Watanabe B, Fujioka S, Hategan L, Ide K, Shibata K, Yokota T, Szekeres M, Mizutani M (2012) CYP90A1/CPD, a brassinosteroid biosynthetic cytochrome P450 of Arabidopsis, catalyzes C-3 oxidation. J Biol Chem 287:31551–31560. doi:10.1074/jbc.M112.392720
Oki K, Inaba N, Kitagawa K, Fujioka S, Kitano H, Fujisawa Y, Kato H, Iwasaki Y (2009) Function of the alpha subunit of rice heterotrimeric G protein in brassinosteroid signaling. Plant Cell Physiol 50:161–172. doi:10.1093/pcp/pcn182
Olszewski N, T-p Sun, Gubler F (2002) Gibberellin signaling biosynthesis, catabolism, and response pathways. Plant Cell 14:S61–S80
Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261
Qiao S, Sun S, Wang L, Wu Z, Li C, Li X, Wang T, Leng L, Tian W, Lu T, Wang X (2017) The RLA1/SMOS1 Transcription Factor Functions with OsBZR1 to Regulate Brassinosteroid Signaling and Rice Architecture. Plant Cell 29:292–309. doi:10.1105/tpc.16.00611
Qi W, Sun F, Wang Q, Chen M, Huang Y, Feng YQ, Luo X, Yang J (2011) Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiol 157:216–228. doi:10.1104/pp.111.179945
Rebetzke G, Ellis M, Bonnett D, Condon A, Falk D, Richards R (2011) The Rht13 dwarfing gene reduces peduncle length and plant height to increase grain number and yield of wheat. Field Crops Res 124:323–331
Rebetzke G, Bonnett D, Ellis M (2012) Combining gibberellic acid-sensitive and insensitive dwarfing genes in breeding of higher-yielding, sesqui-dwarf wheats. Field Crops Res 127:17–25
Ross JJ, Murfet IC, Reid JB (1997) Gibberellin mutants. Physiol Plant 100:550–560
Rutger J, Carnahan H (1981) A fourth genetic element to facilitate hybrid cereal production—a recessive tall in rice. Crop Sci 21:373–376
Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H (2013) The biology of strigolactones. Trends Plant Sci 18:72–83
Saeda T, Kitano H (1992) Genetic analysis of a short culm-mutant showing the dm-type internode distribution pattern. Jpn J Breed 42:214–215
Sakai M, Sakamoto T, Saito T, Matsuoka M, Tanaka H, Kobayashi M (2003) Expression of novel rice gibberellin 2-oxidase gene is under homeostatic regulation by biologically active gibberellins. J Plant Res 116:161–164
Sakamoto T, Matsuoka M (2006) Characterization of constitutive photomorphogenesis and DWARFISM homologs in rice (Oryza sativa L.). J Plant Growth Regul 25:245–251
Sakamoto T, Morinaka Y, Ishiyama K, Kobayashi M, Itoh H, Kayano T, Iwahori S, Matsuoka M, Tanaka H (2003) Genetic manipulation of gibberellin metabolism in transgenic rice. Nat Biotechnol 21:909–913. doi:10.1038/nbt847
Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, Miyao A, Hirochika H, Kitano H, Ashikari M, Matsuoka M (2004) An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol 134:1642–1653. doi:10.1104/pp.103.033696
Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, Ueguchi-Tanaka M, Mizutani M, Sakata K, Takatsuto S, Yoshida S, Tanaka H, Kitano H, Matsuoka M (2006) Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24:105–109. doi:10.1038/nbt1173
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush G (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416:701–702
Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science (New York, N Y) 299:1896–1898. doi:10.1126/science.1081077
Sato Y, Sentoku N, Miura Y, Hirochika H, Kitano H, Matsuoka M (1999) Loss-of-function mutations in the rice homeobox gene OSH15 affect the architecture of internodes resulting in dwarf plants. EMBO J 18:992–1002. doi:10.1093/emboj/18.4.992
Sazuka T, Kamiya N, Nishimura T, Ohmae K, Sato Y, Imamura K, Nagato Y, Koshiba T, Nagamura Y, Ashikari M, Kitano H, Matsuoka M (2009) A rice tryptophan deficient dwarf mutant, tdd1, contains a reduced level of indole acetic acid and develops abnormal flowers and organless embryos. Plant J 60:227–241. doi:10.1111/j.1365-313X.2009.03952.x
Schomburg FM, Bizzell CM, Lee DJ, Zeevaart JA, Amasino RM (2003) Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15:151–163
Sears RG, Kronstad WE, Metzger RJ (1981) Inheritance of dwarf and semi-dwarf plant height in barley. Crop Sci 21:828–833
Shen Z, He Z (1989) Interaction between eui gene and WAMS cytoplasm of rice and improvement of panicle exsertion of MS line. SABRAO J 6:753–756
Shimada Y, Fujioka S, Miyauchi N, Kushiro M, Takatsuto S, Nomura T, Yokota T, Kamiya Y, Bishop GJ, Yoshida S (2001) Brassinosteroid-6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiol 126:770–779
Shimada A, Ueguchi-Tanaka M, Sakamoto T, Fujioka S, Takatsuto S, Yoshida S, Sazuka T, Ashikari M, Matsuoka M (2006) The rice SPINDLY gene functions as a negative regulator of gibberellin signaling by controlling the suppressive function of the DELLA protein, SLR1, and modulating brassinosteroid synthesis. Plant J 48:390–402. doi:10.1111/j.1365-313X.2006.02875.x
Song Y, You J, Xiong L (2009) Characterization of OsIAA1 gene, a member of rice Aux/IAA family involved in auxin and brassinosteroid hormone responses and plant morphogenesis. Plant Mol Biol 70:297–309. doi:10.1007/s11103-009-9474-1
Spielmeyer W, Ellis MH, Chandler PM (2002) Semi-dwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Nati Acad Sci USA 99:9043–9048. doi:10.1073/pnas.132266399
Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2002) The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Nati Acad Sci USA 99:13307–13312. doi:10.1073/pnas.192244099
Sui P, Jin J, Ye S, Mu C, Gao J, Feng H, Shen WH, Yu Y, Dong A (2012) H3K36 methylation is critical for brassinosteroid-regulated plant growth and development in rice. Plant J 70:340–347. doi:10.1111/j.1365-313X.2011.04873.x
Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E (2010) Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev Cell 19:765–777
Sun H, Tao J, Hou M, Huang S, Chen S, Liang Z, Xie T, Wei Y, Xie X, Yoneyama K (2015) A strigolactone signal is required for adventitious root formation in rice. Ann Bot 115:1155–1162
Tabuchi M, Sugiyama K, Ishiyama K, Inoue E, Sato T, Takahashi H, Yamaya T (2005) Severe reduction in growth rate and grain filling of rice mutants lacking OsGS1;1, a cytosolic glutamine synthetase1;1. Plant J 42:641–651. doi:10.1111/j.1365-313X.2005.02406.x
Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, Kato H, Iwasaki Y (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17:776–790. doi:10.1105/tpc.104.024950
Tanaka K, Murata K, Yamazaki M, Onosato K, Miyao A, Hirochika H (2003) Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol 133:73–83
Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, Jiang CJ, Dubouzet JG, Kikuchi S, Sekimoto H, Yokota T, Asami T, Kamakura T, Mori M (2009) Brassinosteroid UPREGULATED1, encoding a helix–loop–helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol 151:669–680. doi:10.1104/pp.109.140806
Thomas SG, Phillips AL, Hedden P (1999) Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Nati Acad Sci USA 96:4698–4703
Tong H, Chu C (2012) Brassinosteroid signaling and application in rice. J Genet Genom 39:3–9. doi:10.1016/j.jgg.2011.12.001
Tong H, Jin Y, Liu W, Li F, Fang J, Yin Y, Qian Q, Zhu L, Chu C (2009) DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J 58:803–816. doi:10.1111/j.1365-313X.2009.03825.x
Toriba T, Harada K, Takamura A, Nakamura H, Ichikawa H, Suzaki T, Hirano HY (2007) Molecular characterization the YABBY gene family in Oryza sativa and expression analysis of OsYABBY1. Mol Genet Genom 277:457–468. doi:10.1007/s00438-006-0202-0
Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M (2000) Rice dwarf mutant d1, which is defective in the alpha subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Nat Acad Sci USA 97:11638–11643. doi:10.1073/pnas.97.21.11638
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YI, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698. doi:10.1038/nature04028
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200. doi:10.1038/nature07272
van Zeijl A, Liu W, Xiao TT, Kohlen W, Yang WC, Bisseling T, Geurts R (2015) The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biol 15:260
Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136:1005–1016. doi:10.1016/j.cell.2009.03.001
Vriet C, Russinova E, Reuzeau C (2012) Boosting crop yields with plant steroids. Plant Cell 24:842–857. doi:10.1105/tpc.111.094912
Vriet C, Russinova E, Reuzeau C (2013) From squalene to brassinolide: the steroid metabolic and signaling pathways across the plant kingdom. Mol Plant 6:1738–1757
Wang L, Xu YY, Ma QB, Li D, Xu ZH, Chong K (2006) Heterotrimeric G protein alpha subunit is involved in rice brassinosteroid response. Cell Res 16:916–922. doi:10.1038/sj.cr.7310111
Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19:63–73. doi:10.1105/tpc.106.048298
Wang L, Xu Y, Zhang C, Ma Q, Joo SH, Kim SK, Xu Z, Chong K (2008) OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PLoS One 3:e3521
Wang ZY, Bai MY, Oh E, Zhu JY (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu Rev Genet 46:701–724. doi:10.1146/annurev-genet-102209-163450
Wang Y, Deng D, Ding H, Xu X, Zhang R, Wang S, Bian Y, Yin Z, Chen Y (2013a) Gibberellin biosynthetic deficiency is responsible for maize dominant Dwarf11 (D11) mutant phenotype: physiological and transcriptomic evidence. PLoS One 8:e66466
Wang Y, Sun S, Zhu W, Jia K, Yang H, Wang X (2013b) Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev Cell 27:681–688. doi:10.1016/j.devcel.2013.11.010
Wang Y, Chen L, Du Y, Yang Z, Condon AG, Hu Y-G (2014) Genetic effect of dwarfing gene Rht13 compared with Rht-D1b on plant height and some agronomic traits in common wheat (Triticum aestivum L.). Field Crops Res 162:39–47
Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA (2012) The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol 159:1073–1085. doi:10.1104/pp.112.196253
Wei XJ, Tang SQ, Shao GN, Chen ML, Hu YC, Hu PS (2013) Fine mapping and characterization of a novel dwarf and narrow-leaf mutant dnl1 in rice. Genet Mol Res 12:3845–3855. doi:10.4238/2013.September.23.2
Wen C, Xi L, Gao B, Wang K, Lv S, Kou Y, Ma N, Zhao L (2015) Roles of DgD14 in regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum ‘Jinba’). Plant Physiol Biochem 96:241–253
Wu CY, Trieu A, Radhakrishnan P, Kwok SF, Harris S, Zhang K, Wang J, Wan J, Zhai H, Takatsuto S, Matsumoto S, Fujioka S, Feldmann KA, Pennell RI (2008) Brassinosteroids regulate grain filling in rice. Plant Cell 20:2130–2145. doi:10.1105/tpc.107.055087
Wu G, Wang X, Li X, Kamiya Y, Otegui MS, Chory J (2011) Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci Signal 4:ra29
Wu Y, Fu Y, Zhao S, Gu P, Zhu Z, Sun C, Tan L (2016) Clustered primary branch1, a new allele of DWARF11, controls panicle architecture and seed size in rice. Plant Biotechnol J 14:377–386. doi:10.1111/pbi.12391
Xie DX, Feys BF, James S, Nietorostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science (New York, N Y) 280:1091–1094
Xu J, Zha M, Li Y, Ding Y, Chen L, Ding C, Wang S (2015) The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Rep 34:1647–1662. doi:10.1007/s00299-015-1815-8
Yaish MW, El-Kereamy A, Zhu T, Beatty PH, Good AG, Bi YM, Rothstein SJ (2010) The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet 6:e1001098. doi:10.1371/journal.pgen.1001098
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12:1591–1606
Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21:2220–2236. doi:10.1105/tpc.109.065730
Yang DL, He SY (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Nati Acad Sci USA 109:1192–1200
Yang RC, Zhang SB, Huang RH, Yang SL, Zhang QQ (2002) Breeding technology of eui-hybrids of rice. Agric Sci China 1:359–363
Yang G, Nakamura H, Ichikawa H, Kitano H, Komatsu S (2006) OsBLE3, a brassinolide-enhanced gene, is involved in the growth of rice. Phytochemistry 67:1442–1454. doi:10.1016/j.phytochem.2006.05.026
Yang C, Shen W, He Y, Tian Z, Li J (2016) OVATE family protein 8 positively mediates brassinosteroid signaling through interacting with the GSK3-like kinase in rice. PLoS Genet 12:e1006118
Yasuno N, Yasui Y, Takamure I, Kato K (2007) Genetic interaction between 2 tillering genes, reduced culm number 1 (rcn1) and tillering dwarf gene d3, in rice. J Heredity 98:169–172. doi:10.1093/jhered/esl069
Yokota T (1997) The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci 2:137–143
Yoshida S, Kameoka H, Tempo M, Akiyama K, Umehara M, Yamaguchi S, Hayashi H, Kyozuka J, Shirasu K (2012) The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytol 196:1208–1216
Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, Guo H, Anderson S, Aluru S, Liu P (2011) A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J 65:634–646
Yuan SJ, Wang T, Yin L, Zhao JG, Wan JM, Li XY (2013) Cloning and expression of gene responsible for high-tillering dwarf phenotype in Indica rice mutant gsor23. Rice Sci 20:320–328
Zazimalova E, Napier RM (2003) Points of regulation for auxin action. Plant Cell Rep 21:625–634. doi:10.1007/s00299-002-0562-9
Zentella R, Sui N, Barnhill B, Hsieh WP, Hu J, Shabanowitz J, Boyce M, Olszewski NE, Zhou P, Hunt DF (2017) The Arabidopsis O-fucosyltransferase SPINDLY activates nuclear growth repressor DELLA. Nat Chem Biol 13:479–485
Zhang LY, Bai MY, Wu J, Zhu JY, Wang H, Zhang Z, Wang W, Sun Y, Zhao J, Sun X, Yang H, Xu Y, Kim SH, Fujioka S, Lin WH, Chong K, Lu T, Wang ZY (2009) Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21:3767–3780. doi:10.1105/tpc.109.070441
Zhang S, Li G, Fang J, Chen W, Jiang H, Zou J, Liu X, Zhao X, Li X, Chu C, Xie Q, Jiang X, Zhu L (2010) The interactions among DWARF10, auxin and cytokinin underlie lateral bud outgrowth in rice. J Integr Plant Biol 52:626–638. doi:10.1111/j.1744-7909.2010.00960.x
Zhang B, Tian F, Tan L, Xie D, Sun C (2011) Characterization of a novel high-tillering dwarf 3 mutant in rice. J Genet Genom 38:411–418. doi:10.1016/j.jgg.2011.08.002
Zhang C, Xu Y, Guo S, Zhu J, Huan Q, Liu H, Wang L, Luo G, Wang X, Chong K (2012) Dynamics of brassinosteroid response modulated by negative regulator LIC in rice. PLoS Genet 8:e1002686. doi:10.1371/journal.pgen.1002686
Zhang C, Bai MY, Chong K (2014a) Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep 33:683–696
Zhang J, Liu X, Li S, Cheng Z, Li C (2014b) The rice semi-dwarf mutant sd37, caused by a mutation in CYP96B4, plays an important role in the fine-tuning of plant growth. PLoS One 9:e88068. doi:10.1371/journal.pone.0088068
Zhang Y, van Dijk AD, Scaffidi A, Flematti GR, Hofmann M, Charnikhova T, Verstappen F, Hepworth J, van der Krol S, Leyser O, Smith SM, Zwanenburg B, Al-Babili S, Ruyter-Spira C, Bouwmeester HJ (2014c) Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat Chem Biol 10:1028–1033. doi:10.1038/nchembio.1660
Zhang X, Song H, Xiaoying C, Rongxiang F (2016) Small G protein as a novel component of the rice bmssinosteroid signal transduction. Mol Plant 9:1260–1271
Zhou HL, He SJ, Cao YR, Chen T, Du BX, Chu CC, Zhang JS, Chen SY (2006) OsGLU1, a putative membrane-bound endo-1,4-beta-d-glucanase from rice, affects plant internode elongation. Plant Mol Biol 60:137–151. doi:10.1007/s11103-005-2972-x
Zhu Y, Nomura T, Xu Y, Zhang Y, Peng Y, Mao B, Hanada A, Zhou H, Wang R, Li P, Zhu X, Mander LN, Kamiya Y, Yamaguchi S, He Z (2006) ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell 18:442–456. doi:10.1105/tpc.105.038455
Zou J, Chen Z, Zhang S, Zhang W, Jiang G, Zhao X, Zhai W, Pan X, Zhu L (2005) Characterizations and fine mapping of a mutant gene for high tillering and dwarf in rice (Oryza sativa L.). Planta 222:604–612. doi:10.1007/s00425-005-0007-0
Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L (2006) The rice high-tillering DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. Plant J 48:687–698. doi:10.1111/j.1365-313X.2006.02916.x
Acknowledgements
Special thanks should go to Dr. Alice Hayward who has put considerable time and effort on improving the clarity and accuracy of this text. This work was supported by a Major Research Project of CAAS Science and the Technology Innovation Program, and by the National Natural Science Foundation of China (Grant numbers: 31400243 and 31201152), and by the Natural Science Foundation of Hubei Province (Grant number: 2013CFB423).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, F., Wang, P., Zhang, X. et al. The genetic and molecular basis of crop height based on a rice model. Planta 247, 1–26 (2018). https://doi.org/10.1007/s00425-017-2798-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2798-1