Abstract
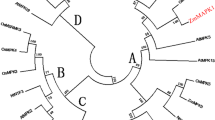
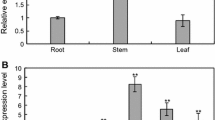
Mitogen-activated protein kinase (MAPK) cascades are involved in biotic and abiotic stress responses. In plants, MAPKs are classified into four groups, designated A–D. Information about group C MAPKs is limited, and, in particular, no data from maize are available. In this article, we isolated a novel group C MAPK gene, ZmMPK7, from Zea mays. Exogenous abscisic acid (ABA) and hydrogen peroxide (H2O2) induced calcium-dependant transcription of ZmMPK7. Induction of this gene in response to ABA was blocked by several reactive oxygen species (ROS) manipulators such as imidazole, Tiron, and dimethylthiourea (DMTU). This result indicates that endogenous H2O2 may be required for ZmMPK7-mediated ABA signaling. Expression of ZmMPK7 in Nicotonia tobaccum caused less H2O2 to accumulate and alleviated ROS-mediated injuries following submission of the plants to osmotic stress. The enhanced total peroxidase (POD) activity in transgenic tobacco plants may contribute to removal of ROS. Finally, we have shown that the ZmMPK7 protein localizes in the nucleus. These results broaden our knowledge regarding plant group C MAPK activity in response to stress signals.






Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- CaMV:
-
Cauliflower mosaic virus
- DMTU:
-
Dimethylthiourea
- DAB:
-
3,3′-Diaminobenzidine
- GFP:
-
Green fluorescent protein
- H2O2 :
-
Hydrogen peroxide
- JA:
-
Jasmonic acid
- MDA:
-
Malondialdehyde
- PAGE:
-
Polyacrylamide gel electrophoresis
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SOD:
-
Superoxide dismutase
References
Agrawal GK, Agrawal SK, Shibato J, Iwahashi H, Rakwal R (2003) Novel rice MAP kinases OsMSRMK3 and OsWJUMK1 involved in encountering diverse environmental stresses and developmental regulation. Biochem Biophys Res Commun 300:775–783
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bakalovic N, Passardi F, Ioannidis V, Cosio C, Penel C, Falquet L, Dunand C (2006) PeroxiBase: a class III plant peroxidase database. Phytochemistry 67:534–539
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Doczi R, Brader G, Pettko-Szandtner A, Rajh I, Djamei A, Pitzschke A, Teige M, Hirt H (2007) The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell 19:3266–3279
Fielding JL, Hall JL (1978) A biochemical and cytological study of peroxidase activity in roots of Pisum sativum. J Exp Bot 29:969–981
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl): 15–45
Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, Ehlting J, Beaudoin N, Barbazuk B, Klessig D, Lee J, Martin G, Mundy J, Ohashi Y, Scheel D, Sheen J, Xing T, Zhang S, Seguin A, Ellis BE (2006) Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci 11:192–198
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hirt H (2000) Connecting oxidative stress, auxin, and cell cycle regulation through a plant mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA 97:2405–2407
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Hu X, Jiang M, Zhang A, Lu J (2005) Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 223:57–68
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24:655–665
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Jiang M, Zhang J (2002a) Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215:1022–1030
Jiang M, Zhang J (2002b) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Jiang M, Zhang J (2003) Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedlings. Plant Cell Environ 26:929–939
Jiang J, Wang P, An G, Wang P, Song CP (2008) The involvement of a P38-like MAP kinase in ABA-induced and H2O2-mediated stomatal closure in Vicia faba L. Plant Cell Rep 27:377–385
Klusener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130:2152–2163
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Lee MO, Cho K, Kim SH, Jeong SH, Kim JA, Jung YH, Shim J, Shibato J, Rakwal R, Tamogami S, Kubo A, Agrawal GK, Jwa NS (2008) Novel rice OsSIPK is a multiple stress responsive MAPK family member showing rhythmic expression at mRNA level. Planta 227:981–990
Liu Q, Xue Q (2007) Computational identification and phylogenetic analysis of the MAPK gene family in Oryza sativa. Plant Physiol Biochem 45:6–14
Group MAPK (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7:301–308
Moore K, Roberts LJ II (1998) Measurement of lipid peroxidation. Free Radic Res 28:659–671
Morris PC (2001) MAP kinase signal transduction pathways in plants. New Phytol 151:67–89
Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96:6553–6557
Ortiz-Masia D, Perez-Amador MA, Carbonell J, Marcote MJ (2007) Diverse stress signals activate the C1 subgroup MAP kinases of Arabidopsis. FEBS Lett 581:1834–1840
Ortiz-Masia D, Perez-Amador MA, Carbonell P, Aniento F, Carbonell J, Marcote MJ (2008) Characterization of PsMPK2, the first C1 subgroup MAP kinase from pea (Pisum sativum L.). Planta 227:1333–1342
Park SY, Ryu SH, Jang IC, Kwon SY, Kim JG, Kwak SS (2004) Molecular cloning of a cytosolic ascorbate peroxidase cDNA from cell cultures of sweet potato and its expression in response to stress. Mol Genet Genomics 271:339–346
Passardi F, Longet D, Penel C, Dunand C (2004) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65:1879–1893
Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406:731–734
Qin N, Olcese R, Bransby M, Lin T, Birnbaumer L (1999) Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc Natl Acad Sci USA 96:2435–2438
Ranieri A, Petacco F, Castagna A, Soldatini GF (2000) Redox state and peroxidase system in sunflower plants exposed to ozone. Plant Sci 159:159–167
Samuel MA, Ellis BE (2002) Double jeopardy: both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell 14:2059–2069
Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, Wisman E (2001) Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 13:113–123
Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y (1995) Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 270:1988–1992
Zhang A, Jiang M, Zhang J, Tan M, Hu X (2006) Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol 141:475–487
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Zwerger K, Hirt H (2001) Recent advances in plant MAP kinase signalling. Biol Chem 382:1123–1131
Acknowledgments
The authors gratefully acknowledge the help from Plant Developmental Biology Laboratory in Shandong Agricultural University, China. This work was supported by the Grants from the Nation Natural Science Foundation of China (No. 30871457, 30471052) and the Program for Changjiang Scholars and Innovative Research Teams in Universities (No. IRT0635).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zong, Xj., Li, Dp., Gu, Lk. et al. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 229, 485–495 (2009). https://doi.org/10.1007/s00425-008-0848-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0848-4