Abstract
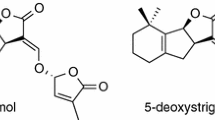
Strigolactones released from plant roots induce hyphal branching of symbiotic arbuscular mycorrhizal (AM) fungi and germination of root parasitic weeds, Striga and Orobanche spp. We already demonstrated that, in red clover plants (Trifolium pratense L.), a host for both AM fungi and the root holoparasitic plant Orobanche minor Sm., reduced supply of phosphorus (P) but not of other elements examined (N, K, Ca, Mg) in the culture medium significantly promoted the secretion of a strigolactone, orobanchol, by the roots of this plant. Here we show that in the case of sorghum [Sorghum bicolor (L.) Moench], a host of both the root hemiparasitic plant Striga hermonthica and AM fungi, N deficiency as well as P deficiency markedly enhanced the secretion of a strigolactone, 5-deoxystrigol. The 5-deoxystrigol content in sorghum root tissues also increased under both N deficiency and P deficiency, comparable to the increase in the root exudates. These results suggest that strigolactones may be rapidly released after their production in the roots. Unlike the situation in the roots, neither N nor P deficiency affected the low content of 5-deoxystrigol in sorghum shoot tissues.





Similar content being viewed by others
Abbreviations
- AM:
-
Arbuscular mycorrhizal
- Mes:
-
4-Morpholineethanesulfonic acid
- LC/MS/MS:
-
High performance liquid chromatography/tandem mass spectrometry
References
Abu Irmaileh BE (1994) Nitrogen reduces branched broomrape (Orobanche ramosa) seed germination. Weed Sci 42:57–60
Akiyama, Hayashi H (2006) Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot 97:925–931
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Awad AA, Sato D, Kusumoto D, Kamioka H, Takeuchi Y, Yoneyama K (2006) Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche, produced by maize, millet and sorghum. Plant Growth Regul 48:221–227
Buee M, Rossignol M, Jauneau A, Ranjeva R, Beard G (2000) The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol Plant Microbe Interact 13:693–698
Cechin I, Press MC (1993) Nitrogen relations of the sorghum-Striga hermonthica host–parasite association: germination, attachment and early growth. New Phytol 124:681–687
Chalot M, Blaudez D, Brun A (2006) Ammonia: a candidate for nitrogen transfer at the mycorrhizal interface. Trends Plant Sci 11:263–266
Cook CE, Whichard LP, Turner B, Wall ME, Egley GH (1966) Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science 154:1189–1190
Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA, McPhail AT (1972) Germination stimulants. II. The structure of strigol––a potent seed germination stimulant for witchweed (Striga lutea Lour.). J Am Chem Soc 94:6198–9199
Epstein E, Bloom JA (2004) Mineral nutrition of plants: principles and perspectives, 2nd edn. Sinauer, Sunderland
Giovannetti M, Sbrana C, Avio L, Citernesi S A, Logi C (1993) Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during pre-infection stages. New Phytol 125:587–593
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gworgwor NA, Weber HC (2003) Arbuscular mycorrhizal fungi–parasite–host interaction for the control of Striga hermonthica (Del.) Benth. in sorghum [Sorghum bicolor (L.) Moench]. Mycorrhiza 13:277–281
Harrison JM (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59:19–42
Hauck C, Muller S, Schildknecht H (1992) A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J Plant Physiol 139:474–478
He HX, Critchley C, Bledsoe C (2003) Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit Rev Plant Sci 22:531–567
Hodge A (2006) Plastic plants and patchy soils. J Exp Bot 57:401–411
Jain R, Foy CL (1992) Nutrient effects on parasitism and germination of Egyptian broomrape (Orobanche aegyptiaca). Weed Technol 6:269–275
Joel DM (2000) The long-term approach to parasitic weeds control: manipulation of specific developmental mechanisms of the parasite. Crop Prot 19:753–758
Joel DM, Steffens JC, Matthews DE (1995) Germination of weedy root parasites. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York, pp 567–597
Joel DM, Hershenhorm J, Eizenberg H, Aly R, Ejeta G, Rich P J, Ransom J K, Sauerborn J, Rubiales D (2007) Biology and management of weedy root parasites. In: Janick J (ed) Horticultural reviews, vol 33. Willey, New York, pp 267–350
Lendzemo VW, Kuyper TW, Kropff MJ, Ast AV (2005) Field inoculation with arbuscular mycorrhizal fungi reduces Striga hermonthica performance on cereal crops and has the potential to contribute to integrated Striga management. Field Crops Res 91:51–61
Marschner H (2002) Mineral nutrition of higher plants, 2nd edn. Academic, California
Müller S, Hauck C, Schildknecht H (1992) Germination stimulants produced by Vigna unguiculata Walp cv. Saunders upright. J Plant Growth Regul 11:77–84
Mumera LM, Below FE (1993) Role of nitrogen in resistance to Striga parasitism of maize. Crop Sci 33:758–763
Muurinen S, Slafer GA, Peltonen-Sainio P (2006) Breeding effects on nitrogen use efficiency of spring cereals under northern conditions. Crop Sci 46:561–568
Nagahashi G, Douds DD (2000) Partial separation of root exudates components and their effects upon the growth of germinated spores of AM fungi. Mycol Res 104:1453–1464
Parker C, Riches R C (1993) Parasitic weeds of the world: biology and control. CAB International, Wallingford
Raghothama GK (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693
Raju PS, Osman MA, Soman P, Peacock JM (1990) Effects of N, P and K on Striga asiatica (L.) Kuntze seed germination and infestation of sorghum. Weed Res 30:139–144
Sato D, Awad AA, Chae SH, Yokota T, Sugimoto Y, Takeuchi Y, Yoneyama K (2003) Analysis of strigolactones, germination stimulants for Striga and Orobanche, by high-performance liquid chromatography/tandem mass spectrometry. J Agric Food Chem 51:1162–1168
Sato D, Awad AA, Takeuchi Y, Yoneyama K (2005) Confirmation and quantification of strigolactones, germination stimulants for root parasitic plants Striga and Orobanche, produced by cotton. Biosci Biotechnol Biochem 69:98–102
Siame BP, Weerasuriya Y, Wood K, Ejeta G, Butler LG (1993) Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J Agric Food Chem 41:1486–1491
Tadano T, Tanaka A (1980) The effect of low phosphate concentrations in culture medium on early growth of several crop plants (in Japanese, translated by the authors). Jpn J Soil Sci Plant Nutr 51:399–404
Taylor TN, Remy W, Hass H, Kerp H (1995) Fossil arbuscular mycorrhizae from the early Devonian. Mycologia 87:560–573
Wang X, Yost RS, Linquist BA (2001) Soil aggregate size affects phosphorus desorption from highly weathered soils and plant growth. Soil Sci Soc Am J 65:139–146
Xu QF, Tsai CL, Tsai CY (1992) Interaction of potassium with the form and amount of nitrogen nutrition on growth and nitrogen uptake of maize. J Plant Nutr 15:23–33
Yokota T, Sakai H, Okuno K, Yoneyama K, Takeuchi Y (1998) Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 49:1967–1973
Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H (2007) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225:1031–1038
Acknowledgments
We acknowledge Dr. Kohki Akiyama (Osaka Prefecture University) for generous gift of synthetic (±)-5-deoxystrigol. We thank Dr. D. M. Joel (Agricultural Research Organization, Ramat Yishay, Israel) for critical reading of the manuscript. A part of this study was supported by the Sasakawa Scientific Research Grant from The Japan Science Society, a Grant-in Aid for Scientific Research (1820810) from Japan Society for the Promotion of Science (JSPS), and a grant for Eminent Research at Utsunomiya University (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoneyama, K., Xie, X., Kusumoto, D. et al. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 227, 125–132 (2007). https://doi.org/10.1007/s00425-007-0600-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0600-5