Abstract
Exercise in the heat causes “central fatigue”, associated with reduced skeletal muscle recruitment during sustained isometric contractions. A similar mechanism may cause fatigue during prolonged dynamic exercise in the heat. The aim of this study was to determine whether centrally regulated skeletal muscle recruitment was altered during dynamic exercise in hot (35°C) compared with cool (15°C) environments. Ten male subjects performed two self-paced, 20-km cycling time-trials, one at 35°C (HOT condition) and one at 15°C (COOL condition). Rectal temperature rose significantly in both conditions, reaching maximum values at 20 km of 39.2±0.2°C in HOT and 38.8±0.1°C in COOL (P<0.005 HOT vs. COOL). Core temperatures at all other distances were not different between conditions. Power output and integrated electromyographic activity (iEMG) of the quadriceps muscle began to decrease early in the HOT trial, when core temperatures, heart rates and ratings of perceived exertion (RPE) were similar in both conditions. iEMG was significantly lower in HOT than in COOL at 10 and 20 km, while power output was significantly reduced in the period from 80% to 100% of the trial duration in the HOT compared with COOL condition. Thus, reduced power output and iEMG activity during self-paced exercise in the heat occurs before there is any abnormal increase in rectal temperature, heart rate or perception of effort. This adaptation appears to form part of an anticipatory response which adjusts muscle recruitment and power output to reduce heat production, thereby ensuring that thermal homeostasis is maintained during exercise in the heat.
Similar content being viewed by others
Introduction
Exercise performance is impaired during both self-paced [16, 35] and externally regulated [5, 7, 25, 26] exercise in the heat. The biological mechanisms explaining this impairment are, however, poorly understood. Originally it was believed that an increase in the oxygen-independent contribution to energy production [3], resulting from a reduction in skeletal muscle blood flow [6, 30] secondary to reduced stroke volume and cardiac output [30], explained this phenomenon.
It is now known, however, that fatigue during exercise in the heat is not caused by reductions in cardiac output or exercising muscle blood flow, or by impaired substrate availability or utilization, or by the accumulation of lactate or K+ [8, 19, 20, 32]. Such fatigue has been observed to occur at a core temperature of approximately 40°C [7, 24], irrespective of the rate of heat storage, the pre-exercise core temperature [8] or the extent of prior heat acclimatization [20, 21]. It has thus been proposed that fatigue during exercise in the heat is associated with a “critical core temperature limiting exercise performance” [8], in which a high body temperature directly affects central nervous functions [19, 24].
Recently, Nybo and Nielsen [24] showed that force production and voluntary activation percentage in the exercised muscle groups (knee extensors) were lower during a sustained isometric maximal voluntary contraction (MVC) following cycle exercise in hot (40°C, sufficient to raise body temperature to 40°C) than in temperate (18°C, final core temperature 38°C) conditions. Significantly, the overall force produced when electrical stimulation was superimposed upon voluntary contraction was unchanged from values measured during the temperate trial. This indicates that the force-generating capacity of the exercised muscle was unaffected by the elevated core and muscle temperatures after exercise in the heat. It was concluded that exercised-induced hyperthermia causes a form of “central fatigue”, in which elevated body temperature (>40°C) caused reduced central activation in the exercised muscles leading to a lower force production. The authors speculated that a similar mechanism exists during dynamic exercise in the heat. To our knowledge, this possibility has yet to be tested.
Accordingly, the aim of this study was to investigate whether centrally-regulated recruitment of skeletal muscle motor units is altered during dynamic exercise in hot (35°C, HOT) compared with cool (15°C, COOL) environments. To evaluate this effect during, as opposed to after the completion of exercise, we studied cyclists during a self-paced, 20-km laboratory cycling time-trial in which they received no verbal or visual feedback other than the distance covered (every kilometre). We have shown previously that this form of testing produces pacing strategies during exercise that are highly reproducible when the testing conditions are identical [33]. We hypothesized that in order to prevent core temperatures from reaching harmful levels during exercise, subjects would subconsciously select a lower power output soon after the start of the time-trial in the HOT compared with the COOL environment, when core temperatures were still significantly lower than levels shown previously to be associated with bodily harm or diminished central drive [8, 19]. Furthermore, it was hypothesized that integrated electromyographic (iEMG) activity in the exercising muscle would be lower in the HOT than in the COOL condition. It has been shown that within an individual, iEMG activity is roughly proportional to the number and diameter of active muscle fibres [1, 9], and iEMG measurements during exercise therefore allow insight into the degree of muscle recruitment and muscle recruitment patterns. A reduction in iEMG activity and power output early on in the HOT condition, before rectal temperatures increase to potentially harmful levels would indicate that skeletal muscle recruitment and power output are down-regulated in advance of thermoregulatory failure. This contrasts with the prevailing hypothesis of fatigue in the heat, which predicts that “central fatigue” develops only after the homeostatic regulation of body temperature has failed and a critical level of hyperthermia is reached.
Materials and methods
Subjects
Ten male cyclists were recruited for the study from local cycling clubs and gymnasia. All subjects were physically active and were fully informed of the risks associated with the study. Subjects signed an informed consent before commencing the study, and upon completion of the trials, were remunerated for their participation. The study was approved by the Research and Ethics Committee of the Faculty of Health Sciences of the University of Cape Town. The mean (±SD) age, height, mass and peak power output (PPO) of the subjects were 24.7±4.6 years, 176.2±6.5 cm, 72.4±8.6 kg and 376±47 W respectively.
Preliminary testing
Subjects reported to the laboratory for preliminary testing consisting of anthropometric measurements and to perform a PPO trial. Stature and body mass were recorded using a precision stadiometer and balance (Model 770, Seca, Bonn, Germany). Percentage body fat was calculated according to the equation of Durnin and Wormersley [4] from skinfold measurements taken at seven sites [29]. Lean thigh volume for each subject was calculated according to the method of Katch and Katch [13], based on the assumption that the thigh is a truncated cone.
PPO was determined on a Kingcycle ergometer (described later) using a modified protocol as described by Hawley and Noakes [10]. Subjects performed a self-paced warm-up for 10 min before beginning the test at a starting power output of 3 W/kg body weight. The workload was increased by 20 W/min until exhaustion. Subjects were required to match a continuously increasing power output displayed in analogue form on the computer monitor. The test was terminated when the subject was unable to match the required power output. PPO was recorded as the highest mean power output achieved over a period of 1 min during the test. Subjects were requested to remain in a seated position throughout the test.
Kingcycle ergometry system
All trials were conducted on an ergometer (Kingcycle, High Wycombe, U.K.) that allows the subjects to ride their own bicycles in the laboratory. After removing the front wheel, the bicycle was firmly secured to the ergometry system by the front fork and supported by an adjustable pillar under the bottom bracket. The bottom bracket support was used to position the rolling resistance of the rear wheel correctly on an air-braked flywheel. A photo-optic sensor monitored the velocity of the flywheel in revolutions per second (RPS), from which an IBM-compatible computer calculated the power output (W) that would be generated by a cyclist riding at that speed on level terrain, using the equation: W=0.000136(RPS)2+1.09(RPS).
The Kingcycle was calibrated before both the peak power output test and the 20-km time-trials. For the calibration, subjects were required to accelerate to a power output of 115 W while seated in their normal cycling position. Once they reached this power output, subjects were instructed to stop pedalling immediately and remain in their riding position. The bottom bracket support was then adjusted until the computer display indicated that the slowing of the flywheel matched a pre-determined reference power decay curve.
Familiarization trial
Within 1 week of the PPO determination, subjects reported to the laboratory for a familiarization trial, during which they became accustomed to the equipment and laboratory conditions for the remaining two trials. Subjects completed a familiarization 20-km time-trial at an ambient temperature of 20°C, relative humidity of 60% and wind velocity of 10 km/h. Subjects were able to drink water ad libitum during the trial. All conditions and procedures were identical to those used in subsequent experimental trials.
Experimental protocol
Within 1 week, subjects reported to the laboratory for the experimental trials, which were conducted in an environmental chamber (Scientific Technology Corporation, Cape Town, South Africa). Each subject performed two experimental 20-km time-trials, one at 35°C (HOT) and one at 15°C (COOL). Relative humidity was 60% and wind speed 10 km/h for both conditions. Five subjects performed the HOT trial first while five performed the COOL trial first. For each subject, trials were conducted at the same time of day so that the effect of circadian variation could be minimized. Trials were separated by between 4–7 days in all subjects to allow sufficient recovery. It was also assumed that subjects were not naturally heat acclimatized as the experiments were conducted between July and October, at which time the outside air temperature ranged from 12–25°C. Subjects were requested not to modify their training for the duration of their involvement in the trial, and to refrain from heavy physical exercise the day before the trial. During trials, subjects were allowed to drink water ad libitum. The only feedback given to subjects during the trials was the elapsed distance at the completion of each kilometre.
Maximal voluntary contraction testing
Prior to each 20-km time-trial, subjects performed a maximal voluntary contraction (MVC) for normalization of the EMG signal obtained during the subsequent trial. The subject’s right knee extensor strength was measured on an isokinetic dynamometer (Kin-Com, Chattanooga Group, USA), while the electromyographic (EMG) activity of the vastus lateralis muscle was recorded. Subjects sat on the dynamometer with their arms folded across their chest and their hips, thighs and upper bodies strapped firmly to the seat. In this position, the hip angle was 100° flexion. The right leg was then attached to the arm of the dynamometer at a level slightly above the lateral malleolus and the axis of rotation of the arm was aligned with the lateral femoral condyle. The arm was then set so that the knee was at a 60° angle from full leg extension. Each subject then performed four sub-maximal familiarization contractions prior to performing four 5-s maximal contractions, separated by 5-s recovery periods. Subjects were encouraged verbally to exert the maximal possible force during each contraction. The contraction producing the highest force was recorded and used for subsequent normalization of the EMG signal obtained during the 20-km time-trial.
EMG testing
During each MVC and subsequent 20-km time-trial, the EMG activity of the vastus lateralis muscle was recorded. Before placement of the electrode, the skin was shaved and cleaned with 95% ethanol, according to methods previously described [14, 34]. A triode electrode (Thought Technology, West Chazy, N.Y., USA) was placed over the muscle belly of the vastus lateralis and connected to a pre-amplifier. The electrode was firmly taped to the skin using micropore tape, and a bandage (Flexwrap) wrapped around the electrode to minimize sweat interference. Outputs from the pre-amplifier were relayed to a Flexcomp/DSP EMG apparatus (Thought Technology, USA) via a fibre-optic cable and stored by an online computer. EMG signals were captured at 1984 Hz during the MVC and the time-trials. EMG activity was captured for 5-s periods during the MVC. During the 20-km time-trials, EMG activity was measured at 1, 5, 10, 15 and 20 km. For analysis of the signal, 5 s of data were analysed because subjects selected their own cadence while cycling. The raw EMG signals were full-wave rectified, movement artefacts removed using a high-pass, second-order Butterworth filter with a cut-off frequency of 15 Hz, then smoothed with a low-pass, second-order Butterworth filter with a cut-off frequency of 5 Hz. This was performed using MATLAB gait analysis software. This iEMG was used for subsequent analysis. All EMG data were normalized by dividing the EMG value obtained at each measurement point during the time-trials by the EMG value obtained during the MVC performed before the start of each time-trial. iEMG data were therefore expressed as a percentage of this MVC data. We have previously shown that this method of EMG normalization is reliable and valid for use in cycling trials, [12] and that the neuromuscular responses (iEMG) during self-paced cycling in the heat are reproducible between trials using this methodology [14]. Because of technical problems with the EMG computer, a complete set of EMG measurements could not be obtained during two of the time-trials and so EMG data from two subjects were rejected and the EMG data reported here represents the results from eight subjects.
Following MVC testing, subjects reported to the environmental chamber and inserted a rectal thermometer (YSI409AC, Yellow Springs Instruments, Yellow Springs, Ohio, USA) 10 cm beyond their anal sphincter. Saltin and Hermansen have shown that the measurement of rectal temperature is as good an index of core temperature during cycling at high work rates as oesophageal temperature [31]. Four surface thermocouples (model 427, YSI) were taped securely to the sternum region, left mid-thigh, left calf and forehead for measurement of skin temperature. Three of these sites (sternum, mid-thigh and calf) are typically measured during temperature-related studies [27], as they measure skin temperature over the heart (sternum thermocouple) and the working skeletal muscle (thigh and calf thermocouples). The forehead site was selected since it was speculated that the temperature of the head may be important in monitoring temperature and regulating exercise intensity, according to the current hypothesis. Upon entering the chamber, the Kingcycle was calibrated as described, and subjects performed a self-paced, 2-min warm-up. The duration of the warm-up was restricted to 2 min to ensure that the initial values for heart rate, rating of perceived exertion (RPE) and core temperature were not different between HOT and COOL conditions. The initial values of skin temperature, rectal temperature and heart rate were obtained at the completion of the warm-up. Temperatures were recorded at 1, 5, 10, 15 and 20 km during the trial using a digital telethermometer, accurate to 0.1°C (YSI 400 series).
Power output during the time-trials was recorded by the Kingcycle equipment. To allow comparisons to be made between power outputs during trials of different duration, the recorded power output was normalized by dividing the trial into intervals of 5% of the total trial duration. Power output is thus reported as the average power output over each of these intervals of 5% total trial duration.
Heart rate was recorded at the start of the trial, and at 1, 5, 10, 15 and 20 km using a Polar Accurex NV heart rate monitor (Polar Electro, Kempele, Finland).
RPE was recorded at the start of the trial and at 1, 5, 10, 15 and 20 km, using the Borg category ratio scale [2].
Subjects recorded their nude body mass before each trial, and again after completion of the trial after wiping off sweat with a towel. The volume of water ingested during each trial was also recorded. Rate of weight loss (in kilograms/hour) was estimated by the change in body mass adjusted for fluid consumption. This weight loss was considered a proxy for sweat rate, but was not corrected for other body weight losses caused by irreversible fuel oxidation, since it was assumed that such losses would be essentially similar in both trials.
Statistical analysis
Power outputs, EMG data, temperatures and heart rate data were analysed using a two-way ANOVA for repeated measures, to examine the interaction of temperature and time. Where a significant effect was detected, post-hoc comparisons were made using Tukey’s “honestly significantly different” (HSD) test for pairwise comparisons. Performance times, average power output, pre and post body weights and fluid ingestion were analysed using a dependent t-test. For all analyses significance was accepted at P<0.05. Data are presented as means±SD.
Results
Time-trial performance and power outputs
The time taken to complete the 20-km trial was significantly greater in the HOT than in the COOL condition (P<0.001) (Table 1). The average power output in HOT was correspondingly lower; 255±47 W compared with 272±45 W in COOL (P<0.01) (Table 1).
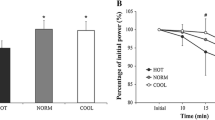
Figure 1 shows the normalized power output recorded during the time-trials, at intervals of 5% of the total duration of the time-trial. Power outputs were identical for the first 30% of the trial duration in both conditions. In HOT, power output declined progressively in the period from 30% to 80%, whereas in COOL, power output remained constant from 10% to 90% of the time-trial. The power output in HOT became significantly lower than in COOL at 80%, 90%, 95% and 100% of the total duration (P<0.05). In both HOT and COOL conditions, the power output in the final 5% interval was significantly greater than in the previous intervals (P<0.05). The highest power output over any 5% interval was recorded in the final 5% in both HOT and COOL conditions.
Thermoregulatory responses
Rectal temperature increased significantly over time (P<0.001) in both HOT and COOL conditions (Fig. 2). In HOT, the final rectal temperature was 39.2±0.6°C, compared with 38.8±0.4°C in COOL (P<0.005). At all other intervals, rectal temperatures were not significantly different between conditions.
Figure 3 shows the rate of increase in rectal temperature calculated at 2-km intervals. There were no significant differences in rate of increase in rectal temperature over any of the intervals. The average rate of rise in rectal temperature in HOT was 0.085±0.030°C/km, compared with 0.070±0.017°C/km in COOL. These were not significantly different.
All four skin temperatures (chest, thigh, calf, forehead) were significantly greater in HOT than in COOL conditions throughout the time-trials (P<0.001) (Fig. 4a–d).
iEMG amplitude
iEMG amplitude, expressed as a percentage of the iEMG amplitude during the MVC prior to each trial, is shown in Fig. 5. iEMG activity was lower in HOT than in COOL at 10 km and 20 km (P<0.05). At 15 km, there was a tendency for iEMG to be lower in HOT than in COOL, but this was not significant (P=0.1). iEMG did not change during the HOT trial, whereas iEMG in COOL at 20 km was significantly greater than at 1, 5, 10 or 15 km (P<0.005).
Heart rates
Heart rate in both conditions increased similarly over time, and final heart rates were significantly greater than at rest (P<0.0001) (Fig. 6). Final heart rates in HOT and COOL were 184±8 and 181±10 bpm respectively. These were not significantly different.
Ratings of perceived exertion
RPE increased significantly over time in both trials (Fig. 7), but were not significantly different between HOT and COOL. The final RPE in HOT and COOL were 9.0±1.5 and 9.6±1.2 respectively.
Fluid intake and weight loss
Body weight changes during the trials, total fluid intakes and rates of weight loss are presented in Table 2. There were no significant differences in pre- and post-trial body weights between conditions, or in changes in body weight during the trials. Fluid intake in HOT was significantly greater than in COOL (P<0.001). Total weight losses and rates of weight loss were not significantly different between conditions.
Discussion
Current models of exercise-related fatigue attribute fatigue or exhaustion to homeostatic failure in one or more organ systems that are crucial for sustained exercise performance [22]. Accordingly, it was originally proposed that fatigue during exercise in the heat developed after a limitation in blood supply, oxygen delivery or fuel utilization had developed. Recent novel studies have shown that such homeostatic failure does not occur, since skeletal muscle blood flow and metabolism do not reach limiting values in the heat [32]. It is now proposed that central [20] neural recruitment of skeletal muscle motor units is reduced when core body temperature rises to “critical” levels [19, 24]. Thus, according to this model, a failure of body temperature regulation causes critical brain and/or core temperatures to be reached. Central fatigue then occurs, as the “hot brain” is no longer able to recruit a sufficient number of motor units to sustain the previous or expected power output.
To our knowledge, we are the first to show that this general model does not explain accurately what actually happens during self-paced exercise in the heat. For we show that when self-paced exercise is performed in the heat, work output and skeletal muscle recruitment are down-regulated early during the trial, before body temperature is significantly elevated, with the result that thermal homeostasis is maintained similarly during exercise in both temperate and hot conditions. The critical findings that support this novel interpretation are the following:
First, despite marked differences in the environmental conditions in which the time-trials were performed, rectal temperatures were not different between HOT and COOL for the first 15 km of the 20 km time-trial (Fig. 2). Rectal temperatures were different at the end of the trials, but did not reach temperatures measured in heatstroke (>41°C) in either condition. Hence, relative thermal homeostasis was maintained during exercise, and the exercise bout was completed without dangerous levels of hyperthermia being reached.
Second, power output began to decline in HOT after only 30% of the total trial duration, and was significantly less than in the cool condition from 80% of the trial duration until the finish (Fig. 1). However, rectal temperatures (Fig. 2) were not different until the final kilometre during the trials, suggesting that the observed reduction in power output in the heat could not have been caused by a higher core temperature acting directly on the active skeletal muscles or the brain to cause fatigue, as has been hypothesized [24]. Indeed, the highest power output in both conditions was achieved during the final 5% of the time-trial, when core temperatures were at their highest. The core temperature during the final kilometre of the trial in the cool condition was in fact significantly greater than the core temperatures in the hot condition at 5 and 10 km, when the power output had begun to decline in that trial. Yet, power output was maintained throughout the cool trial and increased by 20% during the final 10% of the trial (Fig. 1). Hence, an elevated core temperature cannot be the direct cause of the lower power outputs achieved in the hot than in the cool condition. Rather, it appears that power output is decreased in the heat in the absence of any thermal distress.
Third, iEMG activity was reduced significantly at 10 and 20 km in the hot condition. This indicates that the recruitment of motor units was decreased even when core temperatures were below 40°C. In fact, at 10 km, the core temperatures were remarkably similar between conditions (38.4±0.5°C vs. 38.3±0.4°C in HOT and COOL respectively, Fig. 2). Therefore, the reduced skeletal muscle recruitment in the hot trial can also not be explained by the direct effects of a “critical” core temperature producing “central fatigue” in the motor regions of the brain, as has previously been hypothesized [19].
Rather, we propose that the early decline in power output in the absence of any thermoregulatory disturbance in the heat forms part of an anticipatory response in the brain, which mediates a reduction in skeletal muscle recruitment to ensure that the rate of heat production is reduced. This would allow relative thermal homeostasis to be maintained so that exercise can be completed safely without the development of premature fatigue or heat stroke, even in severe environmental conditions.
Indeed, the rate of increase in rectal temperature, a measure of the rate of heat storage, was not significantly different between the hot and cool conditions (Fig. 3). We speculatively interpret this finding to indicate that the reductions in skeletal muscle recruitment and power output in the hot condition may have been mediated to ensure that similar rates of heat accumulation occurred in hot and cool conditions. If this is indeed the case, then the rate of heat storage in the body may provide crucial afferent sensory inputs to a central controller, which adjusts work rate and skeletal muscle motor unit recruitment during exercise in both hot and cool conditions.
Others have reported similar findings to our own. Tatterson et al. have shown a reduction in power output after only 15 min of a 30-min, self-paced cycling time-trial in the heat, even though core temperatures rose at similar rates in a hot and temperate environment [35]. They postulated that the brain is sensitive to the rate of increase in arterial blood temperature, and selects a power output relative to the rate of rise in core temperature. However, they did not measure EMG activity to confirm this hypothesis.
Similarly, Marino et al. have shown that lighter runners outperform heavier runners during a self-paced, 8-km time-trial following 30 min running at 70% peak treadmill speed in hot (35°C) conditions [16]. A significant correlation was also found between the rate of heat storage and body mass during the time-trial, suggesting that the lighter runners store less heat at the same running speed. It was concluded that this reduced rate of heat storage allows the lighter runners to run faster before reaching a limiting rectal temperature [16]. This suggests that the rate of heat storage may contribute to the afferent input responsible for a reduction in work rate in the heat.
Marino et al. [17] have also shown that African runners, who have a lower rate of heat storage at a given running speed than Caucasian runners, were able to outperform the Caucasian runners in hot (35°C), but not in cool (15°C) conditions during an 8-km time-trial. The difference in running speed between the groups in the heat was present from the onset of the time-trial, despite rectal temperatures which were only moderately elevated (~38°C) and not different between groups. It was suggested that the early reduction in running speed in the heat occurs due to an anticipatory exercise response which would “control the exercise work rate by regulating the number of motor units that are recruited or derecruited during prolonged exercise in the heat” [17].
These findings are compatible with the existence of a centrally-regulated pacing strategy that reduces motor command and exercise intensity specifically to prevent excessive heat storage. Presumably, a central controller calculates the optimum rate of heat production and hence heat storage that will allow the self-paced exercise bout to be completed without the development of a harmful level of hyperthermia (Fig. 2) and then regulates skeletal muscle motor unit recruitment to adjust the metabolic rate accordingly. Indeed, Kayser has proposed that the CNS integrates afferent signals from various sources including the heart, muscles, respiratory system and thermoreceptors, and adjusts motor command to protect the integrity of the organism during exercise [15]. This general model for the role of the CNS during exercise is applicable to exercise in the heat.
If this theory is correct, it is not clear how the brain is able to detect the initial increased rate of heat storage in the hot condition. The skin temperatures at all four measured sites were significantly higher in the heat (Fig. 4). The afferent sensory input from the thermoreceptors in the skin must form part of the integrated response which mediates the decreased central recruitment and power output in the heat. That is, a high skin temperature may inform the brain that the capacity for heat dissipation is reduced [18], and so heat production is reduced to prevent body temperature from rising too rapidly to harmful levels.
It has been found that the exercise-induced increase in muscle sympathetic nerve activity (MSNA) is augmented during muscle heating [28]. The increase in MSNA was attributed to the stimulation of mechanically sensitive muscle afferents that are sensitized by heating [28]. We did not measure muscle temperature directly in this study, though it can be expected that muscle temperature would be elevated to similar or greater levels than rectal temperature, based on previous studies of exercise in the heat [31]. Thus, the sensitization of skeletal muscle afferents may play a role in increased signalling to the CNS during exercise in the heat [28]. A “central programmer”, proposed by Ulmer [36], would integrate this and other afferent signals arising from the muscle and peripheral organs and alter movement or force output to optimize performance [36]. This integral control, termed teleoanticipation, would regulate efferent commands based on the afferent feedback from the periphery and knowledge of the “finishing points”. It must also be assumed that all the subjects had prior experience of exercise in the heat, so that any anticipatory reductions in power output may occur as part of a learned response.
The reductions in power output and iEMG activity were not associated with reductions in heart rate (Fig. 6) or RPE (Fig. 7), which were similar at all points during the two trials. The final RPE values were near maximum in both conditions, indicating that subjects performed to their own maximal volitional capacity. It is also significant that power output and iEMG activity were reduced in the heat despite RPE values which were similar and well below maximal in hot and cool conditions. Therefore, the rating of perceived exertion or, alternatively, the conscious sensation of fatigue does not merely track changes in power output, but is related to the central neural processes involved in the maintenance of, in this case, thermal homeostasis. It clearly makes no sense for the conscious perception of effort to be reduced at the same time that power output is decreasing (Fig. 1). The athlete’s natural response to a falling RPE would be to override this effect consciously, thereby increasing the power output, the rate of heat production, and the probability that homeostatic failure would develop. Thus, for thermal homeostasis to be maintained, the central processes responsible for adjusting power output and muscle recruitment must simultaneously increase the conscious perception of exertion, in order to discourage any conscious overriding of this subconscious control.
We also showed that the power output in both the hot and cool conditions increased during the final 5% of the trial, as previously reported [14, 35]. In the present study, the increase in power output was associated with a significant increase in iEMG activity at the end of the cool, but not the hot trial. This difference was possibly due to methodological limitations. The iEMG activity was measured during the final 20 s of the time-trial, whereas power output was averaged over the final 5% of the trial, and thus reflects an average of the final few minutes of the trial. Another limitation of the present study is that the iEMG activity was measured in one muscle, and it is possible that altered skeletal muscle recruitment patterns in the heat, or the relatively small number of subjects may also explain the lack of a significant increase in iEMG activity in the heat.
Nevertheless, the increased iEMG activity in the cool condition indicates that the athletes were able to increase motor unit recruitment at the end of exercise, despite core temperatures, heart rates and RPE values which were significantly higher than at the start of the trial. This suggests that the afferent inputs that mediate the initial decreases in recruitment and power output can be overridden consciously in the maximal effort at the end of the trial, and supports the existence of a system which adjusts exercise performance by altering efferent motor command during exercise [36]. Such a system would ensure that under exercise conditions, a skeletal muscle reserve is maintained. Indeed, we found that approximately 30% of the available motor units were recruited during exercise (Fig. 5) with an increase of up to 45% in the final sprint, similar to our previous findings [11, 34]. Hence, a cardinal feature of prolonged exercise is the presence of motor unit recruitment reserve; a feature which is not always recognized [23].
In conclusion, power output began to fall within the first 30% of a maximal self-paced time-trial in the heat, reaching significance after 80% of the trial had been completed. Skeletal muscle iEMG activity was also reduced from 10 km during the trial in the hot condition. These decreases were not associated with altered rectal temperature, heart rate or perception of effort compared with exercise in the cool, and occurred well before rectal temperature reached 40°C. It is proposed that this response occurs as part of a centrally controlled neural mechanism, which anticipates an abnormal elevation in body temperature, and alters skeletal muscle recruitment to allow completion of the exercise bout whilst thermal homeostasis is maintained. Impaired exercise performance in the heat is thus not the result of a limiting core temperature, but occurs as part of the central regulation of skeletal muscle recruitment, which controls the rate of heat storage, thereby preventing the development of thermoregulatory derangement during self-paced exercise in the heat.
References
Bigland-Ritchie B (1981) EMG/force relations and fatigue of human voluntary contractions. Exerc Sports Sci Rev 11:75–117
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Brown NJ, Stephenson LA, Lister G, Nadel ER (1982) Relative anaerobiosis during heavy exercise in the heat. Fed Proc 41:1677–1682
Durnin JV, Womersley J (1974) Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–97
Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF (1994) Effect of heat stress on muscle energy metabolism during exercise. J Appl Physiol 77:2827–2831
Fink WJ, Costill DL (1975) Leg muscle metabolism during exercise in the heat and cold. Eur J Appl Physiol 34:247–248
Galloway SD, Maughan RJ (1997) Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 29:1240–1249
Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B (1999) Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86:1032–1039
Häkkinen K (1993) Neuromuscular fatigue and recovery in male and female athletes during heavy resistance exercise. Int J Sports Med 14:53–59
Hawley JA, Noakes TD (1992) Peak power output predicts maximal oxygen uptake and performance time in trained cyclists. Eur J Appl Physiol 65:79–83
Hunter AM, St Clair Gibson A, Mbambo Z, Lambert MI, Noakes TD (2002) The effects of heat stress on neuromuscular activity during endurance exercise. Pflugers Arch 444:738–743
Hunter AM, St Clair GA, Lambert M, Noakes TD (2002) Electromyographic (EMG) normalization method for cycle fatigue protocols. Med Sci Sports Exerc 34:857–861
Katch VL, Katch FI (1974) A simple anthropometric method for calculating segmental leg limb volume. Res Q 45:211–214
Kay D, Marino FE, Cannon J, St Clair GA, Lambert MI, Noakes TD (2001) Evidence for neuromuscular fatigue during high-intensity cycling in warm, humid conditions. Eur J Appl Physiol 84:115–121
Kayser B (2003) Exercise starts and ends in the brain. Eur J Appl Physiol 90:411–419
Marino FE, Mbambo Z, Kortekaas E, Wilson G, Lambert MI, Noakes TD, Dennis SC (2000) Advantages of smaller body mass during distance running in warm, humid environments. Pflugers Arch 441:359–367
Marino FE, Lambert MI, Noakes TD (2004) Superior performance of African runners in warm humid but not in cool environmental conditions. J Appl Physiol 96:124–130
Nielsen B (1996) Olympics in Atlanta: a fight against physics. Med Sci Sports Exerc 28:665–668
Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B (1990) Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol 69:1040–1046
Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B (1993) Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol (Lond) 460:467–485
Nielsen B, Strange S, Christensen NJ, Warberg J, Saltin B (1997) Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch 434:49–56
Noakes TD (2000) Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand J Med Sci Sports 10:123–145
Noakes TD, Peltonen JE, Rusko HK (2001) Evidence that a central governor regulates exercise performance during acute hypoxia and hyperoxia. J Exp Biol 204:3225–3234
Nybo L, Nielsen B (2001) Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 91:1055–1060
Nybo L, Nielsen B (2001) Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol 91:2017–2023
Parkin JM, Carey MF, Zhao S, Febbraio MA (1999) Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol 86:902–908
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Ray CA, Gracey KH (1997) Augmentation of exercise-induced muscle sympathetic nerve activity during muscle heating. J Appl Physiol 82:1719–1725
Ross WD, Marfell-Jones MJ (1992) Kinanthropometry. In: MacDougall JD, Wenger HA, Green HJ (eds) The physiological assessment of high performance athletes. Human Kinetics, Champaign
Rowell LB, Marx HJ, Bruce RA, Conn RD, Kusumi F (1966) Reductions in cardiac output, central blood volume, and stroke volume with thermal stress in normal men during exercise. J Clin Invest 45:1801–1816
Saltin B, Hermansen L (1966) Esophageal, rectal and muscle temperature during exercise. J Appl Physiol 21:1757–1762
Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B (1988) Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol 64:649–657
Schabort EJ, Hawley JA, Hopkins WG, Mujika I, Noakes TD (1998) A new reliable laboratory test of endurance performance for road cyclists. Med Sci Sports Exerc 30:1744–1750
St Clair Gibson A, Schabort EJ, Noakes TD (2001) Reduced neuromuscular activity and force generation during prolonged cycling. Am J Physiol 281:R187–R196
Tatterson AJ, Hahn AG, Martin DT, Febbraio MA (2000) Effects of heat stress on physiological responses and exercise performance in elite cyclists. J Sci Med Sport 3:186–193
Ulmer HV (1996) Concept of an extracellular regulation of muscular metabolic rate during heavy exercise in humans by psychophysiological feedback. Experientia 52:416–420
Acknowledgements
Funding for this experiment was provided by Medical Research Council of South Africa, the University of Cape Town Harry Crossley and Nellie Atkinson Staff Research Funds, Discovery Health and the National Research Foundation of South Africa through the THRIP initiative.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tucker, R., Rauch, L., Harley, Y.X. et al. Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflugers Arch - Eur J Physiol 448, 422–430 (2004). https://doi.org/10.1007/s00424-004-1267-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-004-1267-4