Abstract
Purpose
To determine the impact of local muscle heating and cooling on myogenic and proteolytic gene responses following resistance exercise.
Methods
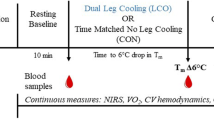
Recreationally trained males (n = 12), age 25.3 ± 1.5, % body fat 13.6 ± 1.92, completed four sets of 8–12 repetitions of unilateral leg press and leg extension while heating one leg, and cooling the other. Muscle biopsies were taken from the vastus lateralis of each leg pre and 4 h post exercise.
Results
MyoD, FOXO1, and MuRF1 mRNA increased with exercise regardless of temperature (p < 0.05). Myostatin, MYF5, and atrogin-1 mRNA decreased with exercise regardless of temperature (p < 0.05). Myogenin, MRF4, and CASP3 mRNA were higher in the hot condition, compared to the cold (p < 0.05). PAX7 mRNA was lower in the hot compared to cold condition (p = 0.041). FOXO3 mRNA was higher in the cold compared to hot condition (p = 0.037). AKT1 and AKT2 were unaffected by either exercise or temperature. Femoral artery blood flow volume was higher in the hot (375.2 ± 41.2 ml min− 1), compared to the cold condition (263.5 ± 23.9 ml min− 1), p = 0.01. Tissue oxygen saturation was higher in the hot (71.7 ± 4.8%) than cold condition (55.3 ± 5.0%).
Conclusion
These results suggest an impaired muscle growth response with local cold application compared to local heat application.



Similar content being viewed by others
Abbreviations
- ACTB:
-
Beta actin
- AKT:
-
Protein kinase B
- ANOVA:
-
Analysis of variance
- ATP:
-
Adenosine triphosphate
- B2M:
-
Beta-2 microglobulin
- CASP3:
-
Caspase 3
- FOXO:
-
Forkhead box O
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- HPRT1:
-
Hypoxanthine phosphoribosyltransferase 1
- MRF:
-
Myogenic regulatory factor
- MRF4:
-
Myogenic regulatory factor 4
- mRNA:
-
Messenger RNA
- MuRF-1:
-
Muscle RING-finger protein-1
- MYF5:
-
Myogenic factor 5
- MyoD:
-
Myogenic differentiation 1
- PAX7:
-
Paired box protein 7
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- RE:
-
Resistance exercise
- RPLP0:
-
Ribosomal protein large 0
- PGC1-α:
-
Peroxisome proliferator-activated receptor 1-alpha
References
Bodine SC, Stitt TN, Gonzalez M et al (2001a) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Bodine SC, Latres E, Baumhueter S et al (2001b) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Braun T, Arnold HH (1995) Inactivation of Myf-6 and Myf-5 genes in mice leads to alterations in skeletal muscle development. EMBO J 14:1176–1186
Coffey VG, Zhong Z, Shield A et al (2006) Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20:190–192
Creer A, Gallagher P, Slivka D, Jemiolo B, Fink W, Trappe S (2005) Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol (1985) 99:950–956
Deldicque L, Atherton P, Patel R et al (2008) Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol 104:57–65
Fujita S, Abe T, Drummond MJ et al (2007) Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol (1985) 103:903–910
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Goyal A, Wang Y, Graham MM, Doseff AI, Bhatt NY, Marsh CB (2002) Monocyte survival factors induce Akt activation and suppress caspase-3. Am J Respir Cell Mol Biol 26:224–230
Gregson W, Black MA, Jones H et al (2011) Influence of cold water immersion on limb and cutaneous blood flow at rest. Am J Sports Med 39:1316–1323
Hamann JJ, Buckwalter JB, Clifford PS, Shoemaker JK (2004) Is the blood flow response to a single contraction determined by work performed? J Appl Physiol (1985) 96:2146–2152
Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ (2006) Human femoral artery and estimated muscle capillary blood flow kinetics following the onset of exercise. Exp Physiol 91:661–671
Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF (1991) Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev Biol 147:144–156
Ijiri D, Kanai Y, Hirabayashi M (2009) Possible roles of myostatin and PGC-1α in the increase of skeletal muscle and transformation of fiber type in cold-exposed chicks: Expression of myostatin and PGC-1α in chicks exposed to cold. Domest Anim Endocrinol 37:12–22
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Kennedy SG, Wagner AJ, Conzen SD et al (1997) The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11:701–713
Kim JS, Cross JM, Bamman MM (2005) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288:E1110-9
Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14:32–43
Lecker SH, Jagoe RT, Gilbert A et al (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Loenneke J, Abe T, Wilson J et al (2012) Blood flow restriction: an evidence based progressive model (review). Acta Physiol Hung 99:235–250
Mammucari C, Schiaffino S, Sandri M (2008) Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4:524–526
Mawhinney C, Jones H, Joo CH, Low DA, Green DJ, Gregson W (2013) Influence of cold-water immersion on limb and cutaneous blood flow after exercise. Med Sci Sports Exerc 45:2277–2285
McKinnell IW, Rudnicki MA (2004) Molecular mechanisms of muscle atrophy. Cell 119:907–910
Naperalsky M, Ruby B, Slivka D (2010) Environmental temperature and glycogen resynthesis. Int J Sports Med 31:561–566
Olguin HC, Olwin BB (2004) Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol 275:375–388
Olguin HC, Yang Z, Tapscott SJ, Olwin BB (2007) Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol 177:769–779
Perry R, Rudnick MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5:148
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273:E99-107
Phillips SM, Tipton KD, Ferrando AA, Wolfe RR (1999) Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol 276:E118-24
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Psilander N, Damsgaard R, Pilegaard H (2003) Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol (1985) 95:1038–1044
Rådegran G (1999) Limb and skeletal muscle blood flow measurements at rest and during exercise in human subjects. Proc Nutr Soc 58:887–898
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 year) and old (80–89 year) women. J Appl Physiol (1985) 101:53–59
Reid MB (2005) Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol 288:R1423-31
Relaix F, Rocancourt D, Mansouri A, Buckingham M (2005) A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435:948–953
Rhodes SJ, Konieczny SF (1989) Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev 3:2050–2061
Rudnicki MA, Le Grand F, McKinnell I, Kuang S (2008) The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73:323–331
Sandri M, Sandri C, Gilbert A et al (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Sandri M, Lin J, Handschin C et al (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protocols 3:1101–1108
Scott BR, Loenneke JP, Slattery KM, Dascombe BJ (2014) Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med 45:313–325
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786
Sharafi H, Rahimi R (2012) The effect of resistance exercise on p53, caspase-9, and caspase-3 in trained and untrained men. J Strength Cond Res 26:1142–1148
Siri WE (1993) Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition 9:480–491
Slivka D, Tucker T, Cuddy J, Hailes W, Ruby B (2012a) Local heat application enhances glycogenesis. Appl Physiol Nutr Metabol 37:247–251
Slivka DR, Dumke CL, Tucker TJ, Cuddy JS, Ruby B (2012b) Human mRNA response to exercise and temperature. Int J Sports Med 33:94–100
Slivka D, Heesch M, Dumke C, Cuddy J, Hailes W, Ruby B (2013) Effects of post-exercise recovery in a cold environment on muscle glycogen, PGC-1α, and downstream transcription factors. Cryobiology 66:250–255
Stitt TN, Drujan D, Clarke BA et al (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Thomas M, Langley B, Berry C et al (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243
Webster KA (1987) Regulation of glycolytic enzyme RNA transcriptional rates by oxygen availability in skeletal muscle cells. Mol Cell Biochem 77:19–28
Welle S, Bhatt K, Thornton CA (1999) Stimulation of myofibrillar synthesis by exercise is mediated by more efficient translation of mRNA. J Appl Physiol (1985) 86:1220–1225
Yang Y, Creer A, Jemiolo B, Trappe S (2005) Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98:1745–1752
Yarasheski KE, Zachwieja JJ, Bier DM (1993) Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 265:E210-4
Zanou N, Gailly P (2013) Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. CMLS Cell Mol Life Sci 70:4117–4130
Zhao J, Brault JJ, Schild A et al (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6:472–483
Acknowledgements
Funding for this project was provided by the University of Nebraska at Omaha Fund for Investing in the Research Enterprise.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Zak, R.B., Hassenstab, B.M., Zuehlke, L.K. et al. Impact of local heating and cooling on skeletal muscle transcriptional response related to myogenesis and proteolysis. Eur J Appl Physiol 118, 101–109 (2018). https://doi.org/10.1007/s00421-017-3749-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3749-z