Abstract
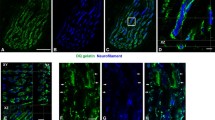
The objective of this study was to investigate the cellular localisation of MyoD and myogenin in human skeletal muscle fibres as well as the possible alterations in the expression of MyoD and myogenin in response to a single bout of endurance exercise at 40% and 75% of maximum oxygen uptake (VO2 max). Twenty-five biopsies (5 per subject) from the vastus lateralis muscle were obtained before exercise, from the exercising leg at 40% and 75% of VO2 max and from the resting leg following these exercise bouts. The tyramide signal amplification-direct and the Vectastain ABC methods using specific monoclonal antibodies were used to determine the exact location of myogenin and MyoD, to identify muscle satellite cells and to determine myosin heavy chain (MyHC) composition. At rest, myonuclei did not express MyoD or myogenin. Following a single bout of exercise at 40% and 75% of VO2 max, an accumulation of myogenin in myonuclei and not in satellite cells was observed in biopsies from the exercised leg but not in biopsies before exercise and from the resting leg. The number of myogenin-positive myonuclei varied among individuals indicating differences in the response to a single exercise bout. In conclusion, this immunohistochemical study showed that a rapid rearrangement of myogenin expression occurs in exercised human skeletal muscles in response to a single bout of exercise.


Similar content being viewed by others
Reference
Buonanno A, Apone L, Morasso MI, Beers R, Brenner HR, Eftimie R (1992) The MyoD family of myogenic factors is regulated by electrical activity: isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Res 20:539–544
Carson JA, Booth FW (1998) Myogenin mRNA is elevated during rapid, slow, and maintenance phases of stretch-induced hypertrophy in chicken slow-tonic muscle. Pflugers Arch 435:850–858
Charifi N, Kadi F, Feasson L, Denis C (2003) Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 28:87–92
Cusella-De Angelis MG, Lyons G, Sonnino C, De Angelis L, Vivarelli E, Farmer K, Wright WE, Molinaro M, Bouche M, Buckingham M, et al (1992) MyoD, myogenin independent differentiation of primordial myoblasts in mouse somites. J Cell Biol 116:1243–1255
Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM (2003) MyoD and myogenin protein expression in skeletal muscles of senile rats. Cell Tissue Res 311:401–416
Dias P, Parham DM, Shapiro DN, Tapscott SJ, Houghton PJ (1992) Monoclonal antibodies to the myogenic regulatory protein MyoD1: epitope mapping and diagnostic utility. Cancer Res 52:6431–6439
Dupont-Versteegden EE, Houle JD, Gurley CM, Peterson CA (1998) Early changes in muscle fiber size and gene expression in response to spinal cord transection and exercise. Am J Physiol 275:1124–1133
Eftimie R, Brenner HR, Buonanno A (1991) Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci U S A 88:1349–1353
Engel ME, Mouton SC, Emms M (1997) Paediatric rhabdomyosarcoma: MyoD1 demonstration in routinely processed tissue sections using wet heat pretreatment (pressure cooking) for antigen retrieval. J Clin Pathol 50:37–39
Eppley ZA, Kim J, Russell B (1993) A myogenic regulatory gene, qmf1, is expressed by adult myonuclei after injury. Am J Physiol 265:397–405
Fuchtbauer EM, Westphal H (1992) MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn 193:34–39
Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW (1992) Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res 267:99–104
Gundersen K (1998) Determination of muscle contractile properties: the importance of the nerve. Acta Physiol Scand 162:333–341
Hespel P, Op’t Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA (2001) Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol 536:625–633
Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter WJ, Peterson CA (1993a) Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development 118:1137–1147
Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM (1993b) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol 158:183–199
Hughes SM, Koishi K, Rudnicki M, Maggs AA (1997) MyoD protein is differentially accumulated in fast and slow skeletal muscle fibres and required for normal fibre type balance in rodents. Mech Dev 61:151–163
Hughes SM, Chi MM, Lowry OH, Gundersen K (1999) Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J Cell Biol 145:633–642
Jacobs-El J, Zhou MY, Russell B (1995) MRF4, Myf-5, and myogenin mRNAs in the adaptive responses of mature rat muscle. Am J Physiol 268:1045–1052
Kadi F (2000) Adaptation of human skeletal muscle to training and anabolic steroids. Acta Physiol Scand 646:1–52
Kadi F, Hagg G, Hakansson R, Holmner S, Butler-Browne GS, Thornell LE (1998) Structural changes in male trapezius muscle with work-related myalgia. Acta Neuropathol Berl 95:352–360
Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE (1999) Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol 111:189–195
Koishi K, Zhang M, Mclennan IS, Harris AJ (1995) MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn 202:244–254
Kraus B, Pette D (1997) Quantification of MyoD, myogenin, MRF4 and Id-1 by reverse transcriptase polymerase chain reaction in rat muscles: effects of hypothyroidism and chronic low-frequency stimulation. Eur J Biochem 247:98–106
Lowe DA, Alway SE (1999) Stretch-induced myogenin, MyoD, and MRF4 expression and acute hypertrophy in quail slow-tonic muscle are not dependent upon satellite cell proliferation. Cell Tissue Res 296:531–539
Lowe DA, Lund T, Alway SE (1998) Hypertrophy-stimulated myogenic regulatory factor mRNA increases are attenuated in fast muscle of aged quails. Am J Physiol 275:155–162
Maier F, Bornemann A (1999) Comparison of the muscle fiber diameter and satellite cell frequency in human muscle biopsies. Muscle Nerve 22:578–583
Molkentin JD, Olson EN (1996) Defining the regulatory networks for muscle development. Curr Opin Genet Dev 6:445–453
Neufer PD, Benjamin IJ (1996) Differential expression of B-crystallin and HSP27 in skeletal muscle during continuous contractile activity. Relationship to myogenic regulatory factors. J Biol Chem 271:24089–24095
Pette D (1998) Training effects on the contractile apparatus. Acta Physiol Scand 162:367–376
Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H (1995) Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72:341–347
Renault V, Thornell LE, Eriksson PO, Butler-Browne GS, Mouly V (2002) Regenerative potential of human skeletal muscle during aging. Aging cell 1:132–139
Rescan PY, Gauvry L, Paboeuf G (1995). A gene with homology to myogenin is expressed in developing myotomal musculature of the rainbow trout and in vitro during the conversion of myosatellite cells to myotubes. FEBS Lett 362:89–92
Schubert W, Zimmermann K, Cramer M, Starzinski-Powitz A (1989) Lymphocyte antigen Leu-19 as a molecular marker of regeneration in human skeletal muscle. Proc Natl Acad Sci U S A 86:307–311
Seward DJ, Haney JC, Rudnicki MA, Swoap SJ (2001) bHLH transcription factor MyoD affects myosin heavy chain expression pattern in a muscle-specific fashion. Am J Physiol 280:408–413
Voytik SL, Przyborski M, Badylak SF, Konieczny SF (1993) Differential expression of muscle regulatory factor genes in normal and denervated adult rat hindlimb muscles. Dev Dyn 198:214–224
Walters EH, Stickland NC, Loughna PT (2000) The expression of the myogenic regulatory factors in denervated and normal muscles of different phenotypes. J Muscle Res Cell Motil 21:647–653
Wang NP, Marx J, Mcnutt MA, Rutledge JC, Gown AM (1995) Expression of myogenic regulatory proteins (myogenin and MyoD1) in small blue round cell tumors of childhood. Am J Pathol 147:1799–1810
Weis J, Kaussen M, Calvo S, Buonanno A (2000) Denervation induces a rapid nuclear accumulation of MRF4 in mature myofibers. Dev Dyn 218:438–451
Wright WE, Binder M, Funk W (1991) Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Mol Cell Biol 11:4104–4110
Wright WE, Dac-Korytko I, Farmer K (1996) Monoclonal antimyogenin antibodies define epitopes outside the bHLH domain where binding interferes with protein–protein and protein–DNA interactions. Dev Genet 19:131–138
Yablonka-Reuveni Z, Seger R, Rivera AJ (1999) Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem 47:23–43
Zhou Z, Bornemann A (2001) MRF4 protein expression in regenerating rat muscle. J Muscle Res Cell Motil 22:311–316
Acknowledgements
This study was supported by grants from the Swedish National Centre for Research in Sports (56/02) and the Loo and Hans Ostermans foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kadi, F., Johansson, F., Johansson, R. et al. Effects of one bout of endurance exercise on the expression of myogenin in human quadriceps muscle. Histochem Cell Biol 121, 329–334 (2004). https://doi.org/10.1007/s00418-004-0630-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-004-0630-z