Abstract
Purpose
In cases of severe retinal diseases, the vitreous body has to be removed and replaced by a suitable biomaterial. Currently, however, no satisfying long-term vitreous substitute is in clinical use. A novel therapeutic concept represents the combination of hyalocytes with suitable biomaterials. The goal of the present study was to evaluate the potential of bFGF and TGF-β1 as tools to control hyalocyte proliferation and the accumulation of extracellular matrix (ECM).
Methods
In vitro investigation on the influence of different concentrations of bFGF and TGF-β1 on hyalocyte morphology, proliferation and ECM production.
Results
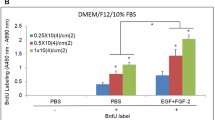
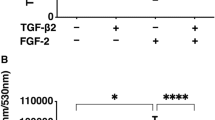
Both growth factors affected hyalocyte morphology; small, round cells could be observed after bFGF supplementation, whereas the cells appeared more completely spread when cultured with TGF-β1. Hyalocyte proliferation was increased 3-fold by 10 ng/ml bFGF; 1 ng/ml TGF-β1 in contrast reduced cell proliferation to about 40% of the control. Converse effects of the growth factors could also be observed on the ECM accumulation of hyalocytes; whereas bFGF halved ECM accumulation, TGF-β1 enhanced the ECM production up to 3-fold. Precultivation of hyalocytes with bFGF for two passages had no influence on their subsequent accumulation of glycosaminoglycans (GAG). However, cells precultivated with bFGF exhibited a doubled accumulation of collagen compared to controls.
Conclusions
The observed opposite effects of bFGF and TGF-β1 on hyalocyte proliferation and ECM accumulation may allow for the control of hyaloycte properties. Therefore, these two growth factors seem to be valuable tools towards the development of a cell-based vitreous substitute.






Similar content being viewed by others
References
Bishop PN (2000) Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res 19:323–344
Bishop PN, Takanosu M, Le Goff M, Mayne R (2002) The role of the posterior ciliary body in the biosynthesis of vitreous humour. Eye 16:454–460
Sebag J (1989) The vitreous. Springer, New York
Henle J (1841) Lehre von den Mischungs- und Formbestandtheilen des menschlichen Körpers, vom Baue des menschlichen Körpers. Vos, Leipzig
Bloom GD, Balazs EA (1965) An electron microscopic study of hyalocytes. Exp Eye Res 4:249–255
Freeman MI, Jacobson B, Balazs EA (1978) The chemical composition of vitreous hyalocytes granules. Exp Eye Res 29:479–484
Gloor BP (1973) Development of the vitreous body and zonula. Graefes Arch Clin Exp Ophthalmol 187:21–44
Gloor BP (1978) Radioisotopes for research into vitreous and zonule. Adv Ophthalmol 36:63–73
Salu P, Claeskens W, De Wilde A, Hijmans W, Wisse E (1985) Light and electron microscopic studies of the rat hyalocyte after perfusion fixation. Ophthalmic Res 17:125–130
Qiao H, Hisatomi T, Sonoda KH, Kura S, Sassa Y, Kinoshita S, Nakamura T, Sakamoto T, Ishibashi T (2005) The characterisation of hyalocytes: the origin, phenotype, and turnover. Br J Ophthalmol 89:513–517
Lazarus HS, Hageman GS (1994) In situ characterization of the human hyalocyte. Arch Ophthalmol 122:1356–1362
Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS (1993) Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci 34:477–487
Boltz-Nitulescu G, Grabner G, Forster O (1979) Macrophage-like properties of human hyalocytes. Adv Exp Med Bio 121B:223–228
Noda Y, Hata Y, Hisatomi T, Nakamura Y, Hirayama K, Miura M, Nakao S, Fujisawa K, Sakamoto T, Ishibashi T (2004) Functional properties of hyalocytes under PDGF-rich conditions. Invest Ophthalmol Vis Sci 45:2107–2114
Zhu M, Penfold PL, Madigan MC, Billson FA (1997) Effect of human vitreous and hyalocyte-derived factors on vascular endothelial cell growth. Aust N Z J Ophthalmol 25:57–60
Badrinath SS, Gopal L, Sharma T, Parikh S, Shanmugam MP, Bhende P, Biswas J (1999) Vitreoschisis in Eales’ disease: pathogenic role and significance in surgery. Retina 19:51–54
Heidenkummer HP, Kampik A (1996) Morphologic analysis of epiretinal membranes in surgically treated idiopathic macular foramina. Results of light and electron microscopy. Der Ophthalmologe 93:675–679
Kobuch K, Wild B, Eckert E, Fischbach C, Gabel VP (2002) Invest Ophthalmol Vis Sci 43: ARVO E-Abstract 3495
Sommer F, Kobuch K, Brandl F, Wild B, Weiser B, Gabel VP, Blunk T, Göpferich A (2007) Ascorbic acid modulates proliferation and ascorbic acid accumulation of hyalocytes. Tissue Eng 13:1281–1289
D’Amico DJ (1994) Diseases of the Retina. N Engl J Med 331:95–106
Frank RN (2004) Diabetic Retinopathy. N Engl J Med 350:48–58
Soman N, Banerjee R (2003) Artificial vitreous replacements. Biomed Mater Eng 13:59–74
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926
Sommer F, Brandl F, Göpferich A (2006) Ocular tissue engineering. Adv Exp Med Biol 585:413–429
Benavente CA, Sierralta WD, Conget PA, Minguell JJ (2003) Subcellular distribution and mitogenic effect of basic fibroblast growth factor in mesenchymal uncommitted stem cells. Growth Factors 21:87–94
Berrada S, Lefebvre F, Harmand MF (1995) The effect of recombinant human basic fibroblast growth factor (rhFGF-2) on human osteoblast in growth and phenotype expression. Dev Biol 31:698–702
Hauner H, Roehrig K, Petruschke T (1995) Effects of epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) on human adipocyte development and function. Eur J Clin Invest 25:90–96
Cuevas P, Burgos J, Baird A (1988) Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem Biophys Res Co 156:611–618
Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R (1997) Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology 138:4456–4462
Martin I, Vunjak-Novakovic G, Yang J, Langer R, Freed LE (1999) Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three- dimensional cartilaginous tissue. Exp Cell Res 253:681–688
Martin I, Suetterlin R, Baschong W, Heberer M, Vunjak-Novakovic G, Freed LE (2001) Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J Cell Biochem 83:121–128
Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y (2001) Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Co 288:413–419
Attisano L, Wrana JL, Lopez-Casillas F, Massague J (1994) TGF-beta receptors and actions. Biochim Biophys Acta 1222:71–80
Massague J (1990) The transforming growth factor-beta family. Ann Rev Cell Biol 6:597–641
Assoian RK, Fleurdelys BE, Stevenson HC, Miller PH, Madtes DK, Raines EW, Ross R, Sporn MB (1987) Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA 84:6020–6024
Cross M, Dexter T (1991) Growth factors in development, transformation, and tumorigenesis. Cell 64:271–280
Kay EP, Lee MS, Seong GJ, Lee YG (1998) TGF-beta stimulates cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr Eye Res 17:286–293
Song QH, Klepeis VE, Nugent MA, Trinkaus-Randall V (2002) TGF-b1 regulates TGF-b1 and FGF-2 mRNA expression during fibroblast wound healing. Mol Pathol 55:164–176
Pangborn CA, Athanasiou KA (2005) Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng 11:1141–1148
Lieb E, Milz S, Vogel T, Hacker M, Dauner M, Schulz MB (2004) Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. I. Culture conditions and tissue formation. Tissue Eng 10:1399–1413
Kee NW, Leong DTW, Hutmacher DW (2002) The challenge to measure cell proliferation in two and three dimensions. Tissue Eng 11:182–191
Kim YJ, Sah RLY, Doong JY, Grodzinsky AJ (1988) Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem 174:168–176
McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL (2002) Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen® and Hoechst 33258. Osteoarthritis Cartilage 10:580–587
Farndale RW, Buttle DJ, Barrett AJ (1986) Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883:173–177
Taylor KB, Jeffree GM (1969) A new basic metachromatic dye, 1,9-dimethyl methylene blue. Histochem J 1:199–204
Woessner JF (1961) The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93:440–447
Chu PK, Chen JY, Wang LP, Huang N (2002) Plasma-surface modification of biomaterials. Mat Sci Eng R 36:143–206
Dickson C, Webster DR, Johnson H, Cecilia Millena A, Khan SA (2002) Transforming growth factor-beta effects on morphology of immature rat Leydig cells. Mol Cell Endocrinol 195:65–77
Stevens MM, Marini RP, Martin I, Langer R, Prasad SV (2004) FGF-2 enhances TGF-b1-induced periosteal chondrogenesis. J Orthop Res 22:1114–1119
Pickering JG, Uniyal S, Ford CM, Chau T, Laurin MA, Chow LH, Ellis CG, Fish J, Chan BM (1997) Fibroblast growth factor-2 potentiates vascular smooth muscle cell migration to platelet-derived growth factor: upregulation of alpha2beta1 integrin and disassembly of actin filaments. Circ Res 80:627–637
Wroblewski J, Edwall-Arvidsson C (1995) Inhibitory effects of basic fibroblast growth factor on chondrocyte differentiation. J Bone Miner Res 10:735–742
Borge L, Lemare F, Demignot S, Adolphe M (1997) Restoration of the differentiated functions of serially passaged chondrocytes using staurosporine. In Vitro Cell Dev Biol Anim 33:703–709
Brown PD, Benya PD (1988) Alterations in chondrocyte cytoskeletal architecture during phenotypic modulation by retinoic acid and dihydrocytochalasin B-induced reexpression. J Cell Biol 106:171–179
Zanetti NC, Solursh M (1984) Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol 99:115–123
Sakamoto T (2003) Cell biology of hyalocytes. Nippon Ganka Gakkai Zasshi 107:866–882
Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB, Göpferich A, Blunk T (2005) Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng 11:1840–1851
Spraul CW, Kaven C, Lang GK, Lang GE (2004) Effect of growth factors on bovine retinal pigment epithelial cell migration and proliferation. Ophthalmic Res 36:166–171
Osterlin SE, Jacobson B (1968) The synthesis of hyaluronic acid in vitreous. II. The presence of soluble transferase and nucleotide sugar in the accellular vitreous gel. Exp Eye Res 7:511–523
Newsome DA, Linsenmayer TF, Trelstad RL (1976) Vitreous body collagen. Evidence for a dual origin from the neural retina and hyalocytes. J Cell Biol 71:59–67
Inoue H, Kato Y, Iwamoto M, Hiraki Y, Sakuda M, Suzuki F (1989) Stimulation of cartilage-matrix proteoglycan synthesis by morphologically transformed chondrocytes grown in the presence of fibroblast growth factor and transforming growth factor-beta. J Cell Physiol 138:329–337
Pei M, Seidel J, Vunjak-Novakovic G, Freed LE (2002) Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Co 294:149–154
Acknowledgement
This work was financially supported by grant 616/04 from the “Bayerische Forschungsstiftung”, Bavaria, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have full control of all primary data and they agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data upon request.
Rights and permissions
About this article
Cite this article
Sommer, F., Pollinger, K., Brandl, F. et al. Hyalocyte proliferation and ECM accumulation modulated by bFGF and TGF-β1. Graefes Arch Clin Exp Ophthalmol 246, 1275–1284 (2008). https://doi.org/10.1007/s00417-008-0846-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0846-z