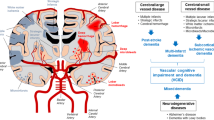
Abstract
Cerebral small vessel disease (CSVD) is a broad category of cerebrovascular diseases which primarily affect the perforating arterioles, capillaries and venules with multiple distinct etiologies. In spite of distinctive pathogenesis, CSVD shares similar neuroimaging markers, including recent small subcortical infarct, lacune of presumed vascular origin, white matter hyperintensity of presumed vascular origin, perivascular space and cerebral microbleeds. The radiological features of neuroimaging markers are indicative for etiological analysis. Furthermore, in sporadic arteriosclerotic pathogenesis associated CSVD, the total CSVD burden is a significant predictor for stroke events, global cognitive impairment, psychiatric disorders and later life quality. This review aims to summarize the radiological characteristics as well as the clinical implication of CSVD markers and neuroimaging interpretation for CSVD symptomatology.


Similar content being viewed by others
References
Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9:689–701
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM (2014) Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83:1228–1234
Li Y, Li M, Zuo L, Shi Q, Qin W, Yang L, Jiang T, Hu W (2018) Compromised blood–brain barrier integrity is associated with total magnetic resonance imaging burden of cerebral small vessel disease. Front Neurol 9:221
Pavlovic AM, Pekmezovic T, Zidverc Trajkovic J, Svabic Medjedovic T, Veselinovic N, Radojicic A, Mijajlovic M, Tomic G, Jovanovic Z, Norton M, Sternic N (2016) Baseline characteristic of patients presenting with lacunar stroke and cerebral small vessel disease may predict future development of depression. Int J Geriatr Psychiatry 31:58–65
Zhang X, Tang Y, Xie Y, Ding C, Xiao J, Jiang X, Shan H, Lin Y, Li C, Hu D, Li T, Sheng L (2017) Total magnetic resonance imaging burden of cerebral small-vessel disease is associated with post-stroke depression in patients with acute lacunar stroke. Eur J Neurol 24:374–380
Liang Y, Chen YK, Deng M, Mok VCT, Wang DF, Ungvari GS, Chu CW, Kamiya A, Tang WK (2017) Association of cerebral small vessel disease burden and health-related quality of life after acute ischemic stroke. Front Aging Neurosci 9:372
Sacco S, Marini C, Totaro R, Russo T, Cerone D, Carolei A (2006) A population-based study of the incidence and prognosis of lacunar stroke. Neurology 66:1335–1338
Moran C, Phan TG, Srikanth VK (2012) Cerebral small vessel disease: a review of clinical, radiological, and histopathological phenotypes. Int J Stroke 7:36–46
Arboix A, Estevez S, Rouco R, Oliveres M, Garcia-Eroles L, Massons J (2015) Clinical characteristics of acute lacunar stroke in young adults. Exp Rev Neurother 15:825–831
Rutten-Jacobs LCA, Markus HS (2017) Vascular risk factor profiles differ between magnetic resonance imaging-defined subtypes of younger-onset lacunar stroke. Stroke 48:2405–2411
Zhu S, McClure LA, Lau H, Romero JR, White CL, Babikian V, Nguyen T, Benavente OR, Kase CS, Pikula A (2015) Recurrent vascular events in lacunar stroke patients with metabolic syndrome and/or diabetes. Neurology 85:935–941
Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J (2013) Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke 44:2995–2999
Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM (2013) Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 382:507–515
Arboix A, Font A, Garro C, Garcia-Eroles L, Comes E, Massons J (2007) Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. J Neurol Neurosurg Psychiatry 78:1392–1394
Traylor M, Bevan S, Baron JC, Hassan A, Lewis CM, Markus HS (2015) Genetic architecture of lacunar stroke. Stroke 46:2407–2412
Neurology Working Group of the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium tSGNS, and the International Stroke Genetics Consortium (ISGC) (2016) Identification of additional risk loci for stroke and small vessel disease: a meta-analysis of genome-wide association studies. Lancet Neurol 15:695–707
Traylor M, Rutten-Jacobs LC, Thijs V, Holliday EG, Levi C, Bevan S, Malik R, Boncoraglio G, Sudlow C, Rothwell PM, Dichgans M, Markus HS (2016) Genetic associations with white matter hyperintensities confer risk of lacunar stroke. Stroke 47:1174–1179
Fisher CM (1991) Lacunar infarct: a review. Cerebrovasc Dis 1:311–320
Arboix A, Lopez-Grau M, Casasnovas C, Garcia-Eroles L, Massons J, Balcells M (2006) Clinical study of 39 patients with atypical lacunar syndrome. J Neurol Neurosurg Psychiatry 77:381–384
Grau-Olivares M, Arboix A, Bartres-Faz D, Junque C (2007) Neuropsychological abnormalities associated with lacunar infarction. J Neurol Sci 257:160–165
Blanco-Rojas L, Arboix A, Canovas D, Grau-Olivares M, Oliva Morera JC, Parra O (2013) Cognitive profile in patients with a first-ever lacunar infarct with and without silent lacunes: a comparative study. BMC neurology 13:203
Bonnin-Vilaplana M, Arboix A, Parra O, Garcia-Eroles L, Montserrat JM, Massons J (2009) Sleep-related breathing disorders in acute lacunar stroke. J Neurol 256:2036–2042
Arboix A, Marti-Vilalta JL (2004) New concepts in lacunar stroke etiology: the constellation of small-vessel arterial disease. Cerebrovasc Dis 17(Suppl 1):58–62
Arboix A, Blanco-Rojas L, Marti-Vilalta JL (2014) Advancements in understanding the mechanisms of symptomatic lacunar ischemic stroke: translation of knowledge to prevention strategies. Expert Rev Neurother 14:261–276
Benavente OR, Pearce LA, Bazan C, Roldan AM, Catanese L, Bhat Livezey VM, Vidal-Pergola G, McClure LA, Hart RG (2014) Clinical-MRI correlations in a multiethnic cohort with recent lacunar stroke: the SPS3 trial. Int J Stroke 9:1057–1064
Valdes Hernandez MD, Qiu X, Wang X, Wiseman S, Sakka E, Maconick LC, Doubal F, Sudlow CL, Wardlaw JM (2017) Interhemispheric characterization of small vessel disease imaging markers after subcortical infarct. Brain Behav 7:e00595
Valdes Hernandez Mdel C, Maconick LC, Munoz Maniega S, Wang X, Wiseman S, Armitage PA, Doubal FN, Makin S, Sudlow CL, Dennis MS, Deary IJ, Bastin M, Wardlaw JM (2015) A comparison of location of acute symptomatic vs. ‘silent’ small vessel lesions. Int J Stroke 10:1044–1050
Duan Z, Fu C, Chen B, Xu G, Tao L, Tang T, Hou H, Fu X, Yang M, Liu Z, Zhang X (2015) Lesion patterns of single small subcortical infarct and its association with early neurological deterioration. Neurol Sci 36:1851–1857
Duan Z, Sun W, Liu W, Xiao L, Huang Z, Cao L, Li H, Xiong Y, Liu D, Xu G, Liu X (2015) Acute diffusion-weighted imaging lesion patterns predict progressive small subcortical infarct in the perforator territory of the middle cerebral artery. Int J Stroke 10:207–212
Arboix A, Garcia-Plata C, Garcia-Eroles L, Massons J, Comes E, Oliveres M, Targa C (2005) Clinical study of 99 patients with pure sensory stroke. J Neurol 252:156–162
Arboix A, Padilla I, Massons J, Garcia-Eroles L, Comes E, Targa C (2001) Clinical study of 222 patients with pure motor stroke. J Neurol Neurosurg Psychiatry 71:239–242
Duering M, Csanadi E, Gesierich B, Jouvent E, Herve D, Seiler S, Belaroussi B, Ropele S, Schmidt R, Chabriat H, Dichgans M (2013) Incident lacunes preferentially localize to the edge of white matter hyperintensities: insights into the pathophysiology of cerebral small vessel disease. Brain 136:2717–2726
Gesierich B, Duchesnay E, Jouvent E, Chabriat H, Schmidt R, Mangin JF, Duering M, Dichgans M (2016) Features and determinants of lacune shape: relationship with fiber tracts and perforating arteries. Stroke 47:1258–1264
Tsai HH, Pasi M, Tsai LK, Chen YF, Lee BC, Tang SC, Fotiadis P, Huang CY, Yen RF, Gurol ME, Jeng JS (2018) Distribution of lacunar infarcts in asians with intracerebral hemorrhage: a magnetic resonance imaging and amyloid positron emission tomography study. Stroke 49:1515–1517
Hong YJ, Kim CM, Kim JE, Roh JH, Kim JS, Seo SW, Na DL, Lee JH (2017) Regional amyloid burden and lacune in pure subcortical vascular cognitive impairment. Neurobiol Aging 55:20–26
Benjamin P, Trippier S, Lawrence AJ, Lambert C, Zeestraten E, Williams OA, Patel B, Morris RG, Barrick TR, MacKinnon AD, Markus HS (2018) Lacunar infarcts, but not perivascular spaces, are predictors of cognitive decline in cerebral small-vessel disease. Stroke 49:586–593
Chen Y, Wang A, Tang J, Wei D, Li P, Chen K, Wang Y, Zhang Z (2015) Association of white matter integrity and cognitive functions in patients with subcortical silent lacunar infarcts. Stroke 46:1123–1126
Reijmer YD, Freeze WM, Leemans A, Biessels GJ (2013) The effect of lacunar infarcts on white matter tract integrity. Stroke 44:2019–2021
Thong JY, Hilal S, Wang Y, Soon HW, Dong Y, Collinson SL, Anh TT, Ikram MK, Wong TY, Venketasubramanian N, Chen C, Qiu A (2013) Association of silent lacunar infarct with brain atrophy and cognitive impairment. J Neurol Neurosurg Psychiatry 84:1219–1225
Makin SD, Turpin S, Dennis MS, Wardlaw JM (2013) Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J Neurol Neurosurg Psychiatry 84:893–900
van Leijsen EMC, Bergkamp MI, van Uden IWM, Ghafoorian M, van der Holst HM, Norris DG, Platel B, Tuladhar AM, de Leeuw FE (2018) Progression of white matter hyperintensities preceded by heterogeneous decline of microstructural integrity. Stroke 49:1386–1393
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 149:351–356
Lampe L, Kharabian-Masouleh S, Kynast J, Arelin K, Steele CJ, Loffler M, Witte AV, Schroeter ML, Villringer A, Bazin PL (2017) Lesion location matters: the relationships between white matter hyperintensities on cognition in the healthy elderly. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X17740501 (Epub ahead of print)
Shim YS, Yang DW, Roe CM, Coats MA, Benzinger TL, Xiong C, Galvin JE, Cairns NJ, Morris JC (2015) Pathological correlates of white matter hyperintensities on magnetic resonance imaging. Dement Geriatr Cogn Disord 39:92–104
Zhang CE, Wong SM, Uiterwijk R, Backes WH, Jansen JFA, Jeukens C, van Oostenbrugge RJ, Staals J (2018) Blood–brain barrier leakage in relation to white matter hyperintensity volume and cognition in small vessel disease and normal aging. Brain Imaging Behav. https://doi.org/10.1007/s11682-018-9855-7 (Epub ahead of print)
Osborn KE, Liu D, Samuels LR, Moore EE, Cambronero FE, Acosta LMY, Bell SP, Babicz MA, Gordon EA, Pechman KR, Davis LT, Gifford KA, Hohman TJ, Blennow K, Zetterberg H, Jefferson AL (2018) Cerebrospinal fluid beta-amyloid42 and neurofilament light relate to white matter hyperintensities. Neurobiol Aging 68:18–25
Scott JA, Braskie MN, Tosun D, Maillard P, Thompson PM, Weiner M, DeCarli C, Carmichael OT (2016) Cerebral amyloid is associated with greater white-matter hyperintensity accrual in cognitively normal older adults. Neurobiol Aging 48:48–52
Valdes Hernandez M, Allerhand M, Glatz A, Clayson L, Munoz Maniega S, Gow A, Royle N, Bastin M, Starr J, Deary I, Wardlaw J (2016) Do white matter hyperintensities mediate the association between brain iron deposition and cognitive abilities in older people? Eur J Neurol 23:1202–1209
Nam KW, Kwon HM, Jeong HY, Park JH, Kim SH, Jeong SM, Yoo TG, Kim S (2017) Cerebral white matter hyperintensity is associated with intracranial atherosclerosis in a healthy population. Atherosclerosis 265:179–183
Squair JW, Field TS, Phillips AA (2018) Journal club: relationship between carotid arterial properties and cerebral white matter hyperintensities. Neurology 90:338–340
Della-Morte D, Dong C, Markert MS, Elkind MSV, Sacco RL, Wright CB, Rundek T (2018) Carotid intima-media thickness is associated with white matter hyperintensities: the Northern Manhattan study. Stroke 49:304–311
Park JH, Kwon HM, Lee J, Kim DS, Ovbiagele B (2015) Association of intracranial atherosclerotic stenosis with severity of white matter hyperintensities. Eur J Neurol 22:44–52, e42–43
Rostanski SK, Zimmerman ME, Schupf N, Manly JJ, Westwood AJ, Brickman AM, Gu Y (2016) Sleep disordered breathing and white matter hyperintensities in community-dwelling elders. Sleep 39:785–791
Cloonan L, Fitzpatrick KM, Kanakis AS, Furie KL, Rosand J, Rost NS (2015) Metabolic determinants of white matter hyperintensity burden in patients with ischemic stroke. Atherosclerosis 240:149–153
Silbert LC, Lahna D, Promjunyakul NO, Boespflug E, Ohya Y, Higashiuesato Y, Nishihira J, Katsumata Y, Tokashiki T, Dodge HH (2018) Risk factors associated with cortical thickness and white matter hyperintensities in dementia free Okinawan elderly. J Alzheimers Dis 63:365–372
Molad J, Kliper E, Korczyn AD, Ben Assayag E, Ben Bashat D, Shenhar-Tsarfaty S, Aizenstein O, Shopin L, Bornstein NM, Auriel E (2017) Only white matter hyperintensities predicts post-stroke cognitive performances among cerebral small vessel disease markers: results from the TABASCO study. J Alzheimers Dis 56:1293–1299
Honningsvag LM, Haberg AK, Hagen K, Kvistad KA, Stovner LJ, Linde M (2018) White matter hyperintensities and headache: a population-based imaging study (HUNT MRI). Cephalalgia. https://doi.org/10.1177/0333102418764891 (Epub ahead of print)
Moon SY, de Souto Barreto P, Rolland Y, Chupin M, Bouyahia A, Fillon L, Mangin JF, Andrieu S, Cesari M, Vellas B (2018) Prospective associations between white matter hyperintensities and lower extremity function. Neurology 90:e1291–e1297
Habes M, Erus G, Toledo JB, Bryan N, Janowitz D, Doshi J, Volzke H, Schminke U, Hoffmann W, Grabe HJ, Wolk DA, Davatzikos C (2018) Regional tract-specific white matter hyperintensities are associated with patterns to aging-related brain atrophy via vascular risk factors, but also independently. Alzheimer’s Dement (Amsterdam, Neth) 10:278–284
Tuladhar AM, Reid AT, Shumskaya E, de Laat KF, van Norden AG, van Dijk EJ, Norris DG, de Leeuw FE (2015) Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke 46:425–432
Kynast J, Lampe L, Luck T, Frisch S, Arelin K, Hoffmann KT, Loeffler M, Riedel-Heller SG, Villringer A, Schroeter ML (2018) White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. J Cereb Blood Flow Metab 38:996–1009
van den Berg E, Geerlings MI, Biessels GJ, Nederkoorn PJ, Kloppenborg RP (2018) White matter hyperintensities and cognition in mild cognitive impairment and Alzheimer’s disease: a domain-specific meta-analysis. J Alzheimers Dis 63:515–527
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Investig 123:1299–1309
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111
Weed LH (1914) Studies on cerebro-spinal fluid. No. III: The pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J Med Res 31:51–91
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212:991–999
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341
Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, Werring DJ (2014) MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry 85:522–525
Shams S, Martola J, Charidimou A, Larvie M, Granberg T, Shams M, Kristoffersen-Wiberg M, Wahlund LO (2017) Topography and determinants of magnetic resonance imaging (MRI)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 6:e006279
Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H (2010) Severity of dilated Virchow–Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 41:2483–2490
Xiao L, Lan W, Sun W, Dai Q, Xiong Y, Li L, Zhou Y, Zheng P, Fan W, Ma N, Guo Z, Chen X, Xie X, Xu L, Zhu W, Xu G, Liu X (2015) Chronic kidney disease in patients with lacunar stroke: association with enlarged perivascular spaces and total magnetic resonance imaging burden of cerebral small vessel disease. Stroke 46:2081–2086
Yang S, Zhang X, Yuan J, Yin J, Hu W (2017) Serum uric acid is independently associated with enlarged perivascular spaces. Sci Rep 7:16435
Duperron MG, Tzourio C, Sargurupremraj M, Mazoyer B, Soumare A, Schilling S, Amouyel P, Chauhan G, Zhu YC, Debette S (2018) Burden of dilated perivascular spaces, an emerging marker of cerebral small vessel disease, is highly heritable. Stroke 49:282–287
Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Jager HR, Werring DJ (2013) Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry 84:624–629
Riba-Llena I, Jimenez-Balado J, Castane X, Girona A, Lopez-Rueda A, Mundet X, Jarca CI, Alvarez-Sabin J, Montaner J, Delgado P (2018) Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke 49:1279–1281
Del Brutto OH, Mera RM (2017) Enlarged perivascular spaces in the basal ganglia are independently associated with intracranial atherosclerosis in the elderly. Atherosclerosis 267:34–38
van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WG, Zwanenburg JJ, Luijten PR, Macklin EA, Rozemuller AJ, Gurol ME, Greenberg SM, Viswanathan A, Martinez-Ramirez S (2016) Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab 36:576–580
Charidimou A, Hong YT, Jager HR, Fox Z, Aigbirhio FI, Fryer TD, Menon DK, Warburton EA, Werring DJ, Baron JC (2015) White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke 46:1707–1709
Hansen TP, Cain J, Thomas O, Jackson A (2015) Dilated perivascular spaces in the Basal Ganglia are a biomarker of small-vessel disease in a very elderly population with dementia. Am J Neuroradiol 36:893–898
Yao M, Zhu YC, Soumare A, Dufouil C, Mazoyer B, Tzourio C, Chabriat H (2014) Hippocampal perivascular spaces are related to aging and blood pressure but not to cognition. Neurobiol Aging 35:2118–2125
Jimenez-Balado J, Riba-Llena I, Garde E, Valor M, Gutierrez B, Pujadas F, Delgado P (2018) Prevalence of hippocampal enlarged perivascular spaces in a sample of patients with hypertension and their relation with vascular risk factors and cognitive function. J Neurol Neurosurg Psychiatry 89:651–656
Zhu YC, Dufouil C, Mazoyer B, Soumare A, Ricolfi F, Tzourio C, Chabriat H (2011) Frequency and location of dilated Virchow–Robin spaces in elderly people: a population-based 3D MR imaging study. Am J Neuroradiol 32:709–713
Bouvy WH, Zwanenburg JJ, Reinink R, Wisse LE, Luijten PR, Kappelle LJ, Geerlings MI, Biessels GJ (2016) Perivascular spaces on 7 T brain MRI are related to markers of small vessel disease but not to age or cardiovascular risk factors. J Cereb Blood Flow Metab 36:1708–1717
Liang Y, Chan YL, Deng M, Chen YK, Mok V, Wang F, Ungvari GS, Chu CW, Tang WK (2018) Enlarged perivascular spaces in the centrum semiovale are associated with poststroke depression: a 3-month prospective study. J Affect Disord 228:166–172
Arba F, Quinn TJ, Hankey GJ, Lees KR, Wardlaw JM, Ali M, Inzitari D (2018) Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int J Stroke 13:47–56
Ding J, Sigurethsson S, Jonsson PV, Eiriksdottir G, Charidimou A, Lopez OL, van Buchem MA, Guethnason V, Launer LJ (2017) Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment susceptibility-Reykjavik study. JAMA Neurol 74:1105–1112
Ishikawa M, Yamada S, Yamamoto K (2018) Dilated perivascular spaces in the centrum semiovale begin to develop in middle age. J Alzheimers Dis 61:1619–1626
Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A, Xuereb J, Jager R, Brandner S, Werring DJ (2014) White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology 82:57–62
Banerjee G, Kim HJ, Fox Z, Jager HR, Wilson D, Charidimou A, Na HK, Na DL, Seo SW, Werring DJ (2017) MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain 140:1107–1116
Laveskog A, Wang R, Bronge L, Wahlund LO, Qiu C (2018) Perivascular spaces in old age: assessment, distribution, and correlation with white matter hyperintensities. Am J Neuroradiol 39:70–76
Lee WJ, Jung KH, Ryu YJ, Kim JM, Lee ST, Chu K, Kim M, Lee SK, Roh JK (2018) Association of cardiac hemodynamic factors with severity of white matter hyperintensities in chronic valvular heart disease. JAMA Neurol 75:80–87
Shams S, Granberg T, Martola J, Charidimou A, Li X, Shams M, Fereshtehnejad SM, Cavallin L, Aspelin P, Wiberg-Kristoffersen M, Wahlund LO (2017) Cerebral microbleeds topography and cerebrospinal fluid biomarkers in cognitive impairment. J Cereb Blood Flow Metab 37:1006–1013
Akoudad S, Portegies ML, Koudstaal PJ, Hofman A, van der Lugt A, Ikram MA, Vernooij MW (2015) Cerebral microbleeds are associated with an increased risk of stroke: the Rotterdam study. Circulation 132:509–516
Laible M, Horstmann S, Mohlenbruch M, Wegele C, Rizos T, Schuler S, Zorn M, Veltkamp R (2015) Renal dysfunction is associated with deep cerebral microbleeds but not white matter hyperintensities in patients with acute intracerebral hemorrhage. J Neurol 262:2312–2322
Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S (2014) Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke 45:1492–1494
Yang Q, Yang Y, Li C, Li J, Liu X, Wang A, Zhao J, Wang M, Zeng X, Fan D (2015) Quantitative assessment and correlation analysis of cerebral microbleed distribution and leukoaraiosis in stroke outpatients. Neurol Res 37:403–409
Chung CP, Chou KH, Chen WT, Liu LK, Lee WJ, Huang AC, Chen LK, Lin CP, Wang PN (2017) Location of cerebral microbleeds and their association with carotid intima-media thickness: a community-based study. Sci Rep 7:12058
Ding L, Hong Y, Peng B (2017) Association between large artery atherosclerosis and cerebral microbleeds: a systematic review and meta-analysis. Stroke Vasc Neurol 2:7–14
Romero JR, Preis SR, Beiser A, DeCarli C, D’Agostino RB, Wolf PA, Vasan RS, Polak JF, Seshadri S (2016) Carotid atherosclerosis and cerebral microbleeds: the Framingham Heart Study. J Am Heart Assoc 5:e002377
Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ (2015) Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study. Arterioscler Thromb Vasc Biol 35:1889–1895
Banerjee G, Wahab KW, Gregoire SM, Jichi F, Charidimou A, Jager HR, Rantell K, Werring DJ (2016) Impaired renal function is related to deep and mixed, but not strictly lobar cerebral microbleeds in patients with ischaemic stroke and TIA. J Neurol 263:760–764
Overbeek EC, Staals J, van Oostenbrugge RJ (2016) Decreased kidney function relates to progression of cerebral microbleeds in lacunar stroke patients. Int J Stroke 11:695–700
Zhang JB, Liu LF, Li ZG, Sun HR, Ju XH (2015) Associations between biomarkers of renal function with cerebral microbleeds in hypertensive patients. Am J Hypertens 28:739–745
Ding J, Sigurdsson S, Garcia M, Phillips CL, Eiriksdottir G, Gudnason V, van Buchem MA, Launer LJ (2015) Risk factors associated with incident cerebral microbleeds according to location in older people: the age, gene/environment susceptibility (AGES)-Reykjavik study. JAMA Neurol 72:682–688
Chung PW, Park KY, Kim JM, Shin DW, Ha SY (2014) Carotid artery calcification is associated with deep cerebral microbleeds. Eur Neurol 72:60–63
Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, Ellis KA, Martins RN, Masters CL, Ames D, Villemagne VL, Rowe CC (2014) Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82:1266–1273
Gregg NM, Kim AE, Gurol ME, Lopez OL, Aizenstein HJ, Price JC, Mathis CA, James JA, Snitz BE, Cohen AD, Kamboh MI, Minhas D, Weissfeld LA, Tamburo EL, Klunk WE (2015) Incidental cerebral microbleeds and cerebral blood flow in elderly individuals. JAMA Neurol 72:1021–1028
Graff-Radford J, Simino J, Kantarci K, Mosley TH Jr, Griswold ME, Windham BG, Sharrett AR, Albert MS, Gottesman RF, Jack CR Jr, Vemuri P, Knopman DS (2017) Neuroimaging correlates of cerebral microbleeds: The ARIC study (Atherosclerosis Risk in Communities). Stroke 48:2964–2972
Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW (2016) Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 73:934–943
Chung CP, Chou KH, Chen WT, Liu LK, Lee WJ, Chen LK, Lin CP, Wang PN (2016) Strictly lobar cerebral microbleeds are associated with cognitive impairment. Stroke 47:2497–2502
Marti-Fabregas J, Delgado-Mederos R, Granell E, Morenas Rodriguez E, Marin Lahoz J, Dinia L, Carrera D, Perez de la Ossa N, Sanahuja J, Sobrino T, De Arce AM, Alonso de Lecinana M (2013) Microbleed burden and hematoma expansion in acute intracerebral hemorrhage. Eur Neurol 70:175–178
Tsivgoulis G, Zand R, Katsanos AH, Turc G, Nolte CH, Jung S, Cordonnier C, Fiebach JB, Scheitz JF, Klinger-Gratz PP, Oppenheim C, Goyal N, Safouris A, Mattle HP, Alexandrov AW, Schellinger PD, Alexandrov AV (2016) Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol 73:675–683
Charidimou A, Imaizumi T, Moulin S, Biffi A, Samarasekera N, Yakushiji Y, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Hernandez-Guillamon M, Montaner J, Casolla B, Gregoire SM, Kang DW, Kim JS, Naka H, Smith EE, Viswanathan A, Jager HR, Al-Shahi Salman R, Greenberg SM, Cordonnier C, Werring DJ (2017) Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta-analysis. Neurology 89:820–829
Lau KK, Lovelock CE, Li L, Simoni M, Gutnikov S, Kuker W, Mak HKF, Rothwell PM (2018) Antiplatelet treatment after transient ischemic attack and ischemic stroke in patients with cerebral microbleeds in 2 large cohorts and an updated systematic review. Stroke 6:1434–1442
Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, Imaizumi T, Fluri F, Naka H, Horstmann S, Veltkamp R, Rothwell PM, Kwa VI, Thijs V, Lee YS, Kim YD, Huang Y, Wong KS, Jager HR, Werring DJ (2016) Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology 87:1501–1510
Lau KK, Wong YK, Teo KC, Chang RSK, Tse MY, Hoi CP, Chan CY, Chan OL, Cheung RHK, Wong EKM, Kwan JSK, Hui ES, Mak HKF (2017) Long-term prognostic implications of cerebral microbleeds in chinese patients with ischemic stroke. J Am Heart Assoc 6:e007360. https://doi.org/10.1161/JAHA.117.007360
Charidimou A, Boulouis G, Shams S, Calvet D, Shoamanesh A (2017) Intracerebral haemorrhage risk in microbleed-positive ischaemic stroke patients with atrial fibrillation: preliminary meta-analysis of cohorts and anticoagulation decision schema. J Neurol Sci 378:102–109
Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, Lip GYH, Cohen H, Banerjee G, Houlden H, White MJ, Yousry TA, Harkness K, Flossmann E, Smyth N, Shaw LJ, Warburton E, Muir KW, Jager HR, Werring DJ (2018) Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 17:539–547
Romero JR, Preis SR, Beiser A, Himali JJ, Shoamanesh A, Wolf PA, Kase CS, Vasan RS, DeCarli C, Seshadri S (2017) Cerebral microbleeds as predictors of mortality: the Framingham Heart Study. Stroke 48:781–783
Romero JR, Beiser A, Himali JJ, Shoamanesh A, DeCarli C, Seshadri S (2017) Cerebral microbleeds and risk of incident dementia: the Framingham Heart Study. Neurobiol Aging 54:94–99
Xu X, Chan QL, Hilal S, Goh WK, Ikram MK, Wong TY, Cheng CY, Chen CL, Venketasubramanian N (2017) Cerebral microbleeds and neuropsychiatric symptoms in an elderly Asian cohort. J Neurol Neurosurg Psychiatry 88:7–11
van Norden AG, van Uden IW, de Laat KF, Gons RA, Kessels RP, van Dijk EJ, de Leeuw FE (2013) Cerebral microbleeds are related to subjective cognitive failures: the RUN DMC study. Neurobiol Aging 34:2225–2230
Banerjee G, Jang H, Kim HJ, Kim ST, Kim JS, Lee JH, Im K, Kwon H, Lee JM, Na DL, Seo SW, Werring DJ (2018) Total MRI small vessel disease burden correlates with cognitive performance, cortical atrophy, and network measures in a memory clinic population. J Alzheimers Dis 63:1485–1497
Cummings JL (1993) Frontal-subcortical circuits and human behavior. Arch Neurol 50:873–880
De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MM (2002) Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 52:335–341
Filley CM (1998) The behavioral neurology of cerebral white matter. Neurology 50:1535–1540
van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786
Tuladhar AM, Lawrence A, Norris DG, Barrick TR, Markus HS, de Leeuw FE (2017) Disruption of rich club organisation in cerebral small vessel disease. Hum Brain Mapp 38:1751–1766
Lawrence AJ, Zeestraten EA, Benjamin P, Lambert CP, Morris RG, Barrick TR, Markus HS (2018) Longitudinal decline in structural networks predicts dementia in cerebral small vessel disease. Neurology 90:e1898–e1910
Loos CMJ, Makin SDJ, Staals J, Dennis MS, van Oostenbrugge RJ, Wardlaw JM (2018) Long-term morphological changes of symptomatic lacunar infarcts and surrounding white matter on structural magnetic resonance imaging. Stroke 49:1183–1188
Hatate J, Miwa K, Matsumoto M, Sasaki T, Yagita Y, Sakaguchi M, Kitagawa K, Mochizuki H (2016) Association between cerebral small vessel diseases and mild parkinsonian signs in the elderly with vascular risk factors. Parkinson Relat Disord 26:29–34
van der Holst HM, van Uden IW, Tuladhar AM, de Laat KF, van Norden AG, Norris DG, van Dijk EJ, Esselink RA, Platel B, de Leeuw FE (2015) Cerebral small vessel disease and incident parkinsonism: the RUN DMC study. Neurology 85:1569–1577
de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE (2011) Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 134:73–83
van der Holst HM, Tuladhar AM, Zerbi V, van Uden IWM, de Laat KF, van Leijsen EMC, Ghafoorian M, Platel B, Bergkamp MI, van Norden AGW, Norris DG, van Dijk EJ, Kiliaan AJ, de Leeuw FE (2018) White matter changes and gait decline in cerebral small vessel disease. Neuroimage Clin 17:731–738
Kim YJ, Kwon HK, Lee JM, Cho H, Kim HJ, Park HK, Jung NY, San Lee J, Lee J, Jang YK, Kim ST, Lee KH, Choe YS, Kim YJ, Na DL, Seo SW (2016) Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology 86:1199–1207
Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ (2004) Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord 19:630–640
Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA (2018) Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev 90:164–173
Brookes RL, Herbert V, Lawrence AJ, Morris RG, Markus HS (2014) Depression in small-vessel disease relates to white matter ultrastructural damage, not disability. Neurology 83:1417–1423
Taylor WD, Aizenstein HJ, Alexopoulos GS (2013) The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 18:963–974
Hollocks MJ, Lawrence AJ, Brookes RL, Barrick TR, Morris RG, Husain M, Markus HS (2015) Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 138:3803–3815
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We declare that we have no conflicts of interest to this work.
Ethical standards
The manuscript does not contain any clinical studies or animal experiments.
Rights and permissions
About this article
Cite this article
Chen, X., Wang, J., Shan, Y. et al. Cerebral small vessel disease: neuroimaging markers and clinical implication. J Neurol 266, 2347–2362 (2019). https://doi.org/10.1007/s00415-018-9077-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9077-3